The title here is from an article on metalenses[cite]10.1021/acs.nanolett.6b01897[/cite] which caught my eye. Metalenses are planar and optically thin layers which can be manufactured using a single-step lithographic process. This contrasts with traditional lenses that are not flat and where the optical properties result from very accurately engineered curvatures, which in turn are expensive to manufacture.
Recollect the suggestion that diazomethane has hypervalent character[cite]10.1039/C5SC02076J[/cite]. When I looked into this, I came to the conclusion that it probably was mildly hypervalent, but on carbon and not nitrogen. Here I try some variations with substituents to see what light if any this casts.
In the previous post, I referred to a recently published review on hypervalency[cite]10.1039/C5SC02076J[/cite] which introduced a very simple way (the valence electron equivalent γ) of quantifying the effect. Diazomethane was cited as one example of a small molecule exhibiting hypervalency (on nitrogen) by this measure.
A recently published review on hypervalency[cite]10.1039/C5SC02076J[/cite] introduced a very simple way of quantifying the effect. One of the molecules which was suggested to be hypervalent using this method was diazomethane. Here I take a closer look.
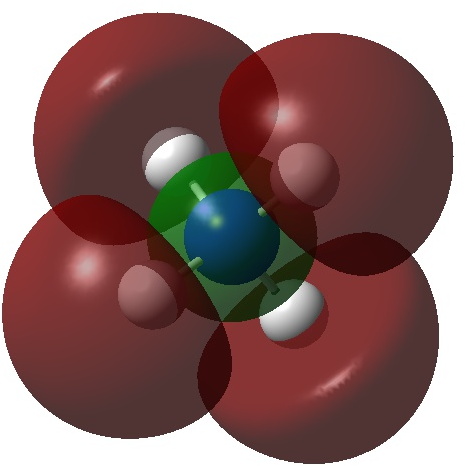
Alkalides are anionic alkali compounds containing e.g. sodide (Na–), kalide (K–), rubidide (Rb–) or caeside (Cs–). Around 90 examples can be found in the Cambridge structure database (see DOI: 10.14469/hpc/3453 for the search query and results). So what about the ammonium analogue, ammonide (NH4–)? A quick search of Scifinder drew a blank!
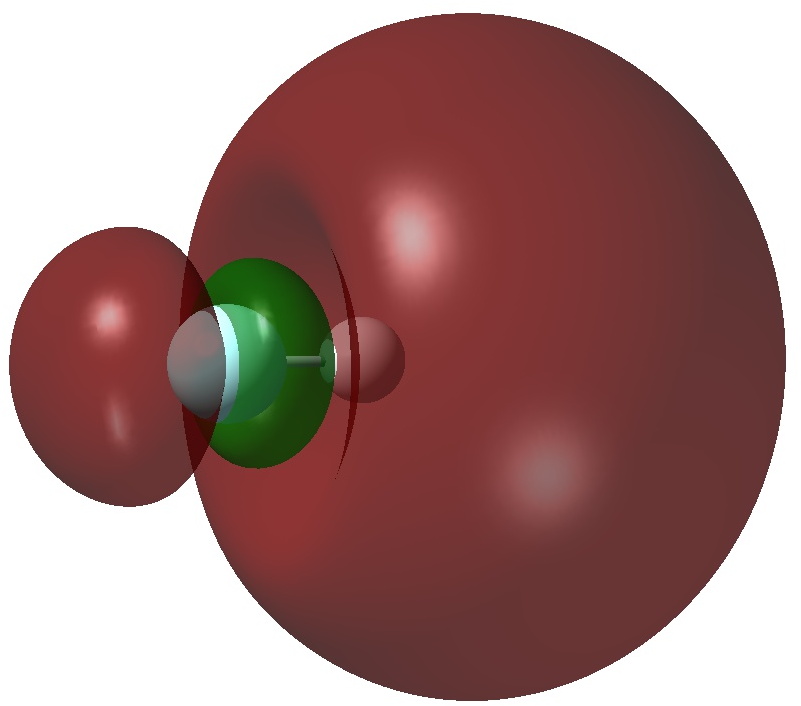
An article with the title shown above in part recently appeared.[cite]10.1038/s41598-017-02687-z[/cite] Given the apparent similarity of HF 1- to CH 3 F 1- and CH 3 F 2- , the latter of which I introduced on this blog previously, I thought it of interest to apply my analysis to HF 1- . The authors[cite]10.1038/s41598-017-02687-z[/cite] conclude that “ the F atom of HF −
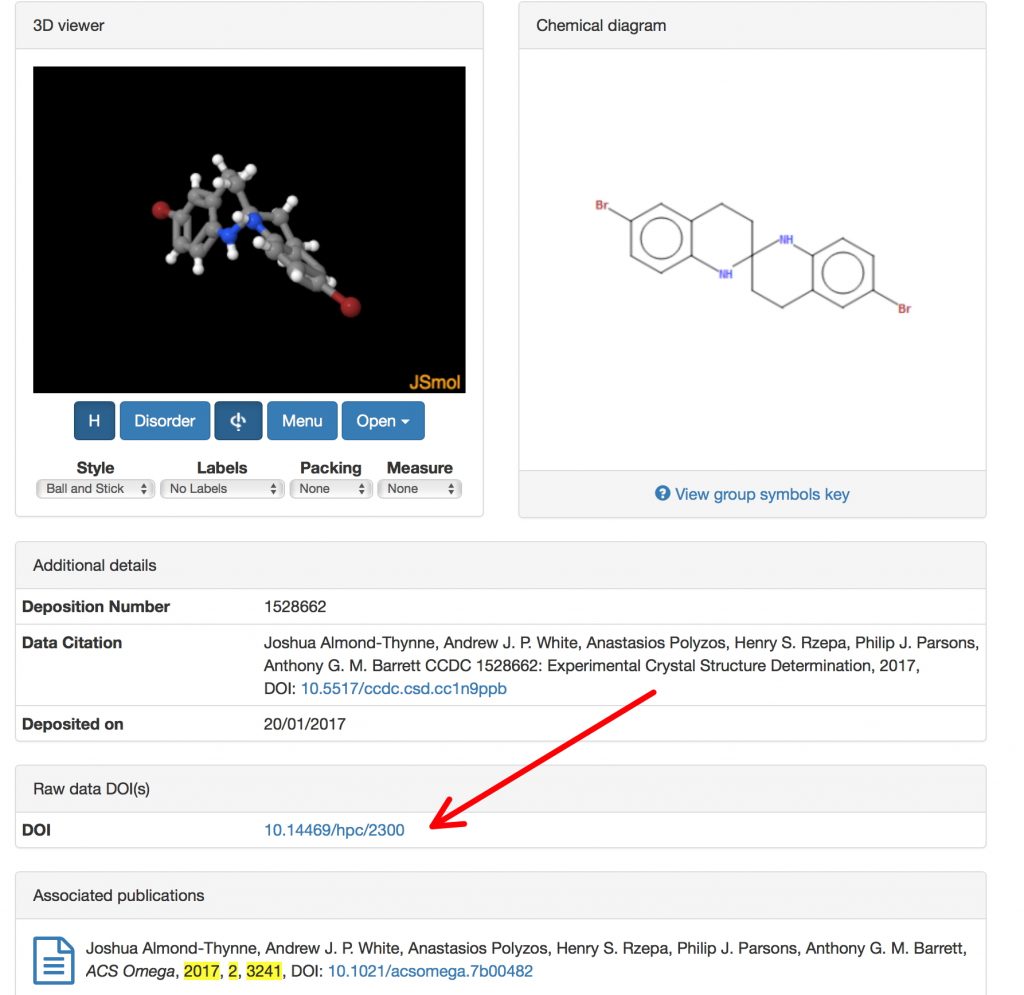
FAIR data is increasingly accepted as a description of what research data should aspire to; **F**indable, **A**ccessible, **I**nter-operable and R e-usable, with Context added by rich metadata (and also that it should be Open). But there are two sides to data, one of which is the raw data emerging from say an instrument or software simulations and the other in which some kind of model is applied to produce semi-
For around 16 years, Floyd Romesberg’s group has been exploring un-natural alternatives (UBPs) to the Watson-Crick base pairs (C-G and A-T) that form part of the genetic code in DNA.
I started this story by looking at octet expansion and hypervalence in non-polar hypercoordinate species such as S(-CH 3 ) 6 , then moved on to S(=CH 2 ) 3 . Finally now its the turn of S(≡CH) 2 . ‡ As the triple bonds imply, this seems to represent twelve shared valence electrons surround the sulfur, six from S itself and three from each carbon.
Previously: “Non-polar” species such as SeMe6, SMe6, ClMe3, ClMe5 all revealed interesting properties for the Se-C, S-C or Cl-C “single” bonds. The latter two examples in particular hinted at internal structures for these single bonds, as manifested by two ELF basins for some of the bonds.
