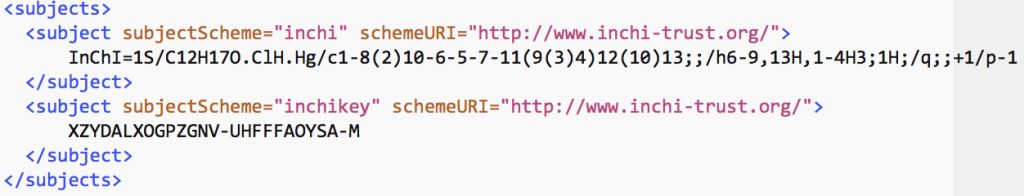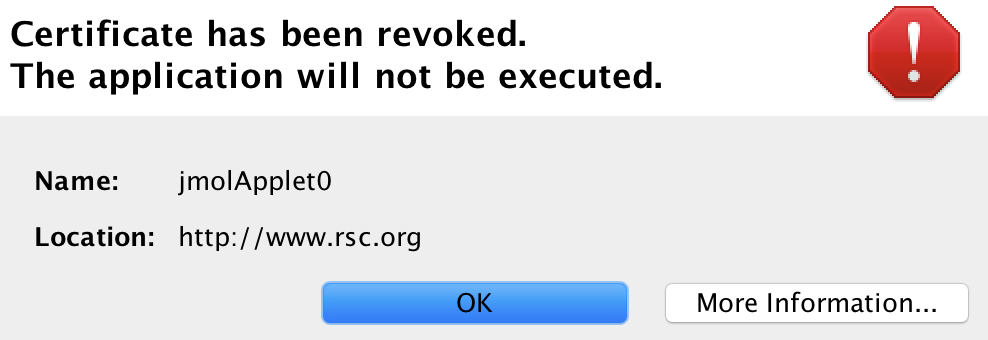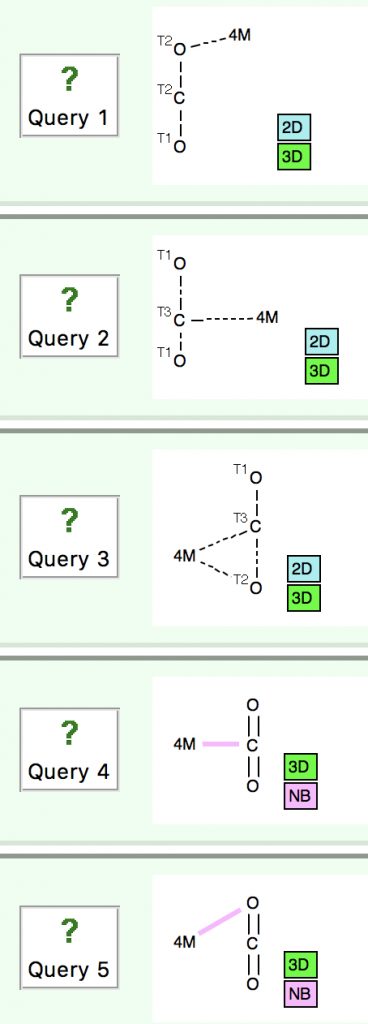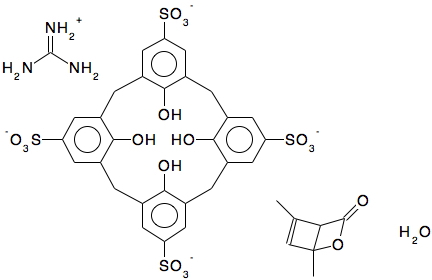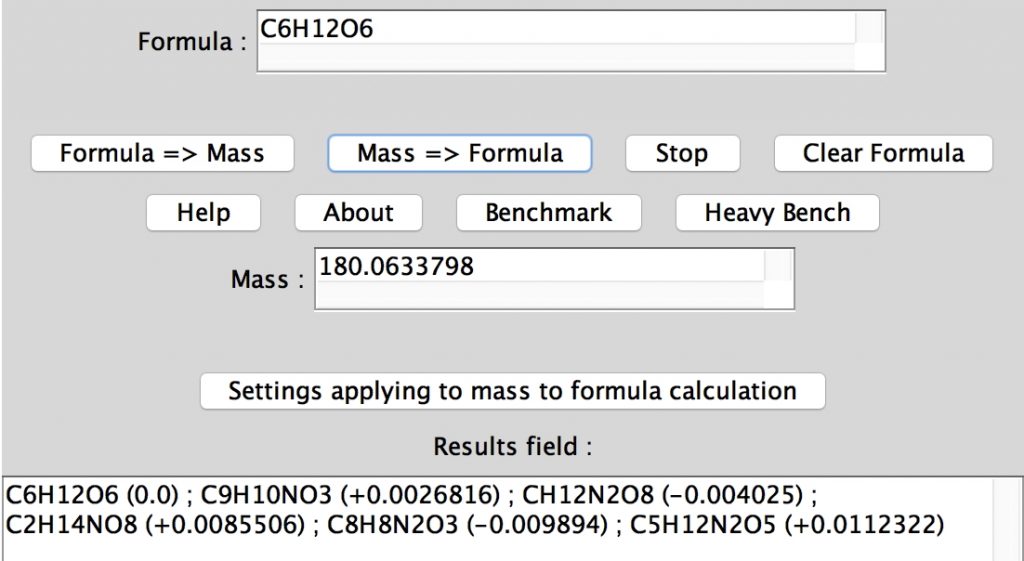
In an earlier post, I lamented the modern difficulties in running old instances of Jmol, an example of an application program written in the Java programming language. When I wrote that, I had quite forgotten a treasure trove of links to old Java that I had collected in 1996-7 and then abandoned.