My PhD thesis involved determining kinetic isotope effects (KIE) for aromatic electrophilic substitution reactions in an effort to learn more about the nature of the transition states involved.[cite]10.1039/p29750001209[/cite] I learnt relatively little, mostly because a transition state geometry is defined by 3N-6 variables (N = number of atoms) and its force constants by even more and you get only one or two measured KIE per reaction;
I am on a mission to persuade my colleagues that the statistical analysis of crystal structures is a useful teaching tool.
As I have noted elsewhere, Gilbert N. Lewis wrote a famous paper entitled “ the atom and the molecule ”, the centenary of which is coming up.[cite]10.1021/ja02261a002[/cite] In a short and rarely commented upon remark, he speculates about the shared electron pair structure of acetylene, R-X≡X-R (R=H, X=C). It could, he suggests, take up three forms. H-C:::C-H and two more which I show as he drew them.
A lunchtime conversation with a colleague had us both bemoaning the distorting influence on chemistry of bibliometrics, h-indices and journal impact factors, all very much a modern phenomenon of scientific publishing. Young academics on a promotion fast-track for example are apparently advised not to publish in a well-known journal devoted to organic chemistry because of its apparently “low” impact factor.

The Bürgi–Dunitz angle is one of those memes that most students of organic chemistry remember. It hypothesizes the geometry of attack of a nucleophile on a trigonal unsaturated (sp 2 ) carbon in a molecule such as ketone, aldehyde, ester, and amide carbonyl.
Blogging in chemistry remains something of a niche activity, albeit with a variety of different styles. The most common is commentary or opinion on the scientific literature or conferencing, serving to highlight what their author considers interesting or important developments. There are even metajournals that aggregate such commentaries. The question therefore occasionally arises;
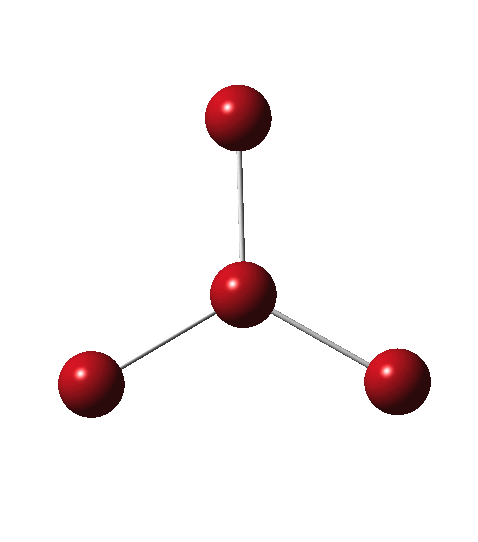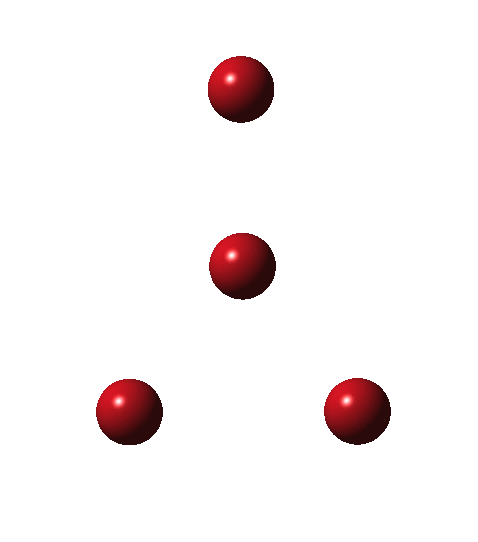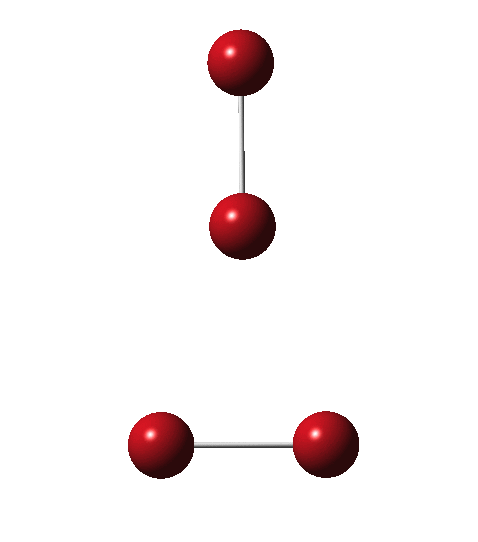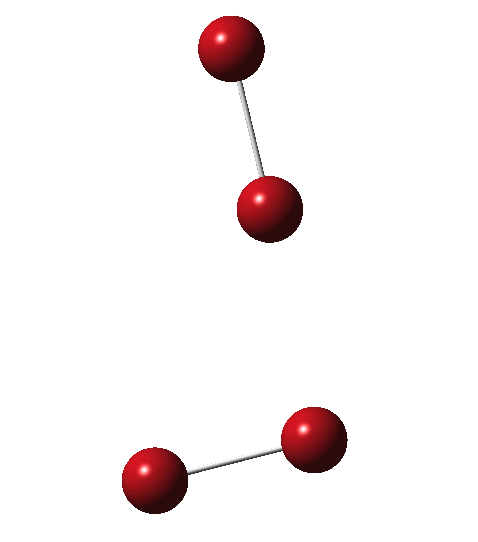
Allotropes are differing structural forms of the elements. The best known example is that of carbon, which comes as diamond and graphite, along with the relatively recently discovered fullerenes and now graphenes. Here I ponder whether any of the halogens can have allotropes. Firstly, I am not aware of much discussion on the topic.

Egon has reminded us that adoption of ORCID (Open researcher and collaborator ID) is gaining apace. It is a mechanism to disambiguate (a Wikipedia term!) contributions in the researcher community and to also remove much of the anonymity (where that is undesirable) that often lurks in social media sites.
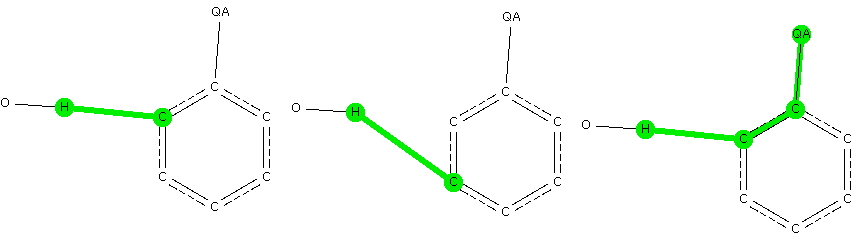
The knowledge that substituents on a benzene ring direct an electrophile engaged in a ring substitution reaction according to whether they withdraw or donate electrons is very old.[cite]10.1039/CT8875100258[/cite] Introductory organic chemistry tells us that electron donating substituents promote the ortho and para positions over the meta . Here I try to recover some of this information by searching crystal structures.
Sodium borohydride is the tamer cousin of lithium aluminium hydride (LAH). It is used in aqueous solution to e.g. reduce aldehydes and ketones, but it leaves acids, amides and esters alone. Here I start an exploration of why it is such a different reducing agent. Initially, I am using Li, not Na (X=Li), to enable a more or less equal comparison with LAH, with water molecules to solvate rather than ether (n=2,3,5) and R set to Me.
