The previous post explored the structural features of amides. Here I compare the analysis with that for the closely related thioamides.

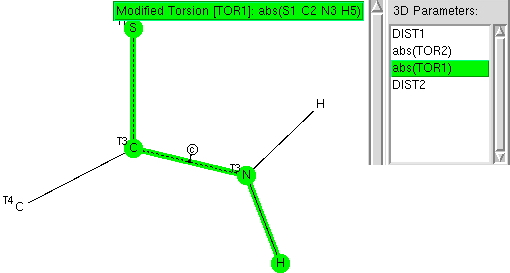
The previous post explored the structural features of amides. Here I compare the analysis with that for the closely related thioamides.
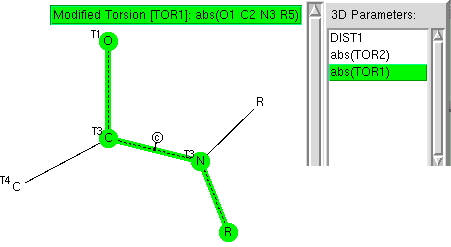
The π-resonance in amides famously helped Pauling to his proposal of a helical structure for proteins. Here I explore some geometric properties of amides related to the C-N bond and the torsions about it.
The first conference devoted to scientific uses of Wikipedia has just finished; there was lots of fascinating stuff but here I concentrate on one report that I thought was especially interesting. To introduce it, I need first to introduce WikiData. This is part of the WikiMedia ecosystem, and one of the newest.

Most visitors to London use the famous underground trains (the “tube”) or a double-decker bus to see the city (one can also use rivers and canals). So I thought, during the tourism month of August, I would show you an alternative overground circumnavigation of the city using the metaphor of benzene.
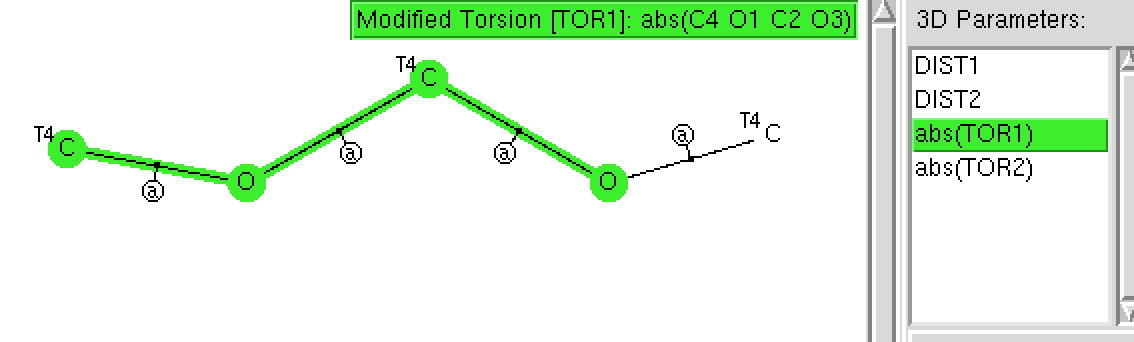
The anomeric effect is best known in sugars, occuring in sub-structures such as RO-C-OR. Its origins relate to how the lone pairs on each oxygen atom align with the adjacent C-O bonds. When the alignment is 180°, one oxygen lone pair can donate into the C-O σ* empty orbital and a stabilisation occurs.
Previously, I showed how conjugation in dienes and diaryls can be visualised by inspecting bond lengths as a function of torsions. Here is another illustration, this time of the mesomeric resonance on a benzene ring induced by an electron donating substituent (an amino group) or an electron withdrawing substituent (cyano).
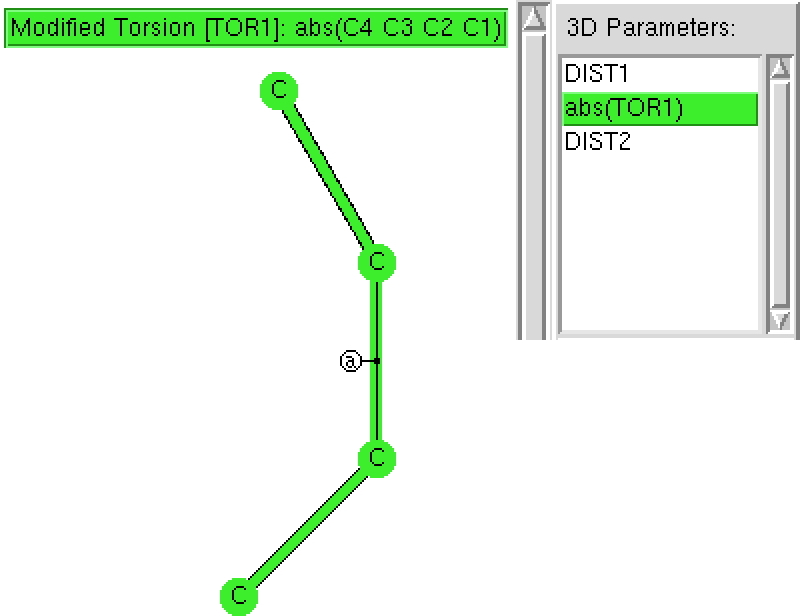
Here is another exploration of simple chemical concepts using crystal structures. Consider a simple diene: how does the central C-C bond length respond to the torsion angle between the two C=C bonds?
Management of research (data) outputs is a hot topic in the UK at the moment, although the topic has been rumbling for five years or more.

This post is prompted by the appearance of a retrospective special issue of C&E news, with what appears to be its very own Website: internet.cenmag.org.
I recently received two emails each with a subject line new approaches to research reporting. The traditional 350 year-old model of the (scientific) journal is undergoing upheavals at the moment with the introduction of APCs (article processing charges), a refereeing crisis and much more. Some argue that brand new thinking is now required.

I recently followed this bloggers trail; link1 → link2 to arrive at this delightful short commentary on atom-atom bonds in crystals by Jack Dunitz. Here he discusses that age-old question (to chemists), what is a bond? Even almost 100 years after Gilbert Lewis’ famous analysis, we continue to ponder this question.