In the beginning (taken here as prior to ~1980) libraries held five-year printed consolidated indices of molecules, organised by formula or name (Chemical abstracts). This could occupy about 2m of shelf space for each five years. And an equivalent set of printed volumes from the Beilstein collection.
In the previous posts, I explored reactions which can be flipped between two potential (stereochemical) outcomes. This triggered a memory from Alex, who pointed out this article from 1999[cite]10.1070/MC1999v009n02ABEH000995[/cite] in which the nitrosonium cation as an electrophile can have two outcomes A or B when interacting with the electron-rich 2,3-dimethyl-2-butene.
This post, the fifth in the series, comes full circle. I started off by speculating how to invert the stereochemical outcome of an electrocyclic reaction by inverting a bond polarity. This led to finding transition states for BOTH outcomes with suitable substitution, and then seeking other examples.
One thing leads to another. Thus in the previous post, I described a thermal pericyclic reaction that appears to exhibit two transition states resulting in two different stereochemical outcomes.
In my first post on the topic, I discussed how inverting the polarity of the C-X bond from X=O to X=Be (scheme below) could flip the stereochemical course of the electrocyclic pericyclic reaction of a divinyl system.
In my earlier post on the topic, I discussed how inverting the polarity of the C-X bond from X=O to X=Be could flip the stereochemical course of the electrocyclic pericyclic reaction of a divinyl system. An obvious question would be: what happens at the half way stage, ie X=CH 2 ? Well, here is the answer.
I do go on a lot about the importance of having modern access to data. And so the appearance of this article[cite]10.1038/sdata.2014.22[/cite] immediately struck me as important. It is appropriately enough in the new journal Scientific Data . The data contain computed properties at the B3LYP/6-31G(2df,p) level for 133,885 species with up to nine heavy atoms, and the entire data set has its own DOI[cite]10.6084/m9.figshare.978904[/cite].
The outcome of pericyclic reactions con depend most simply on three conditions, any two of which determine the third. Whether the catalyst is Δ or hν (heat or light), the topology determining any stereochemistry and the participating electron count (4n+2/4n). It is always neat to conjure up a simple switch to toggle these; heat or light is simple, but what are the options for toggling the electron count?
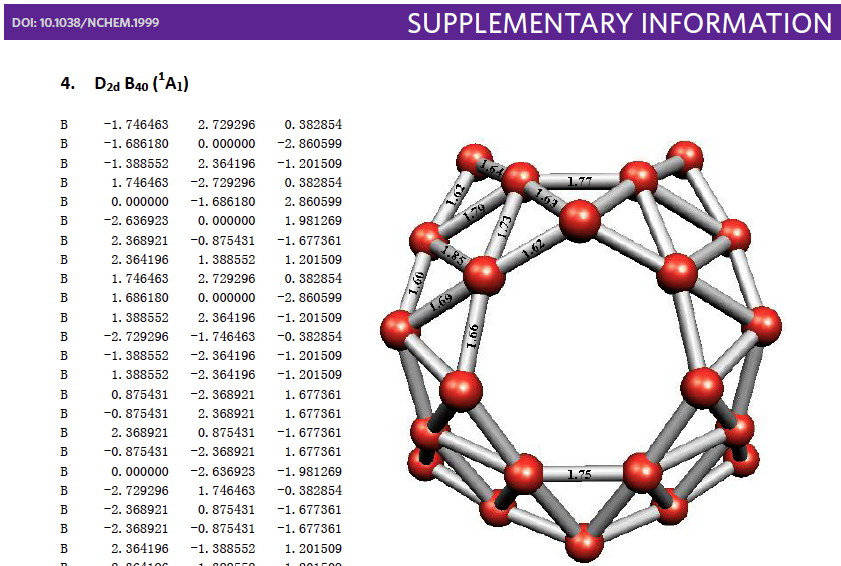
Whilst clusters of carbon atoms are well-known, my eye was caught by a recent article describing the detection of a cluster of boron atoms, B 40 to be specific.[cite]10.1038/nchem.1999[/cite] My interest was in how the σ and π-electrons were partitioned. In a C 40 , one can reliably predict that each carbon would contribute precisely one π-electron. But boron, being more electropositive, does not always play like that.
Computational quantum chemistry has made fantastic strides in the last 30 years. Often deep insight into all sorts of questions regarding reactions and structures of molecules has become possible. But sometimes the simplest of questions can prove incredibly difficult to answer. One such is how accurately can the boiling point of water be predicted from first principles? Or its melting point?
