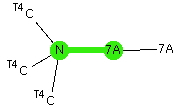
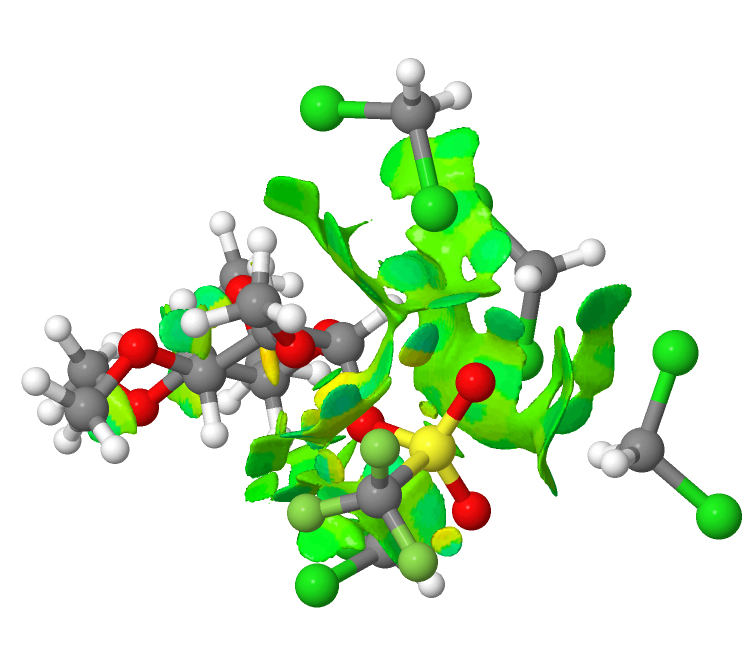
Pursuing the topic of halogen bonds, the system DABCO (a tertiary dibase) and iodine form an intriguing complex. Here I explore some unusual features of the structure HEKZOO[cite]10.5517/CCYJN03[/cite] as published in 2012[cite]10.1021/cg300669t[/cite] and ask whether the bonding between the donor (N) and the acceptor (I-I) really is best described as a “non-covalent-interaction” (NCI) or not.