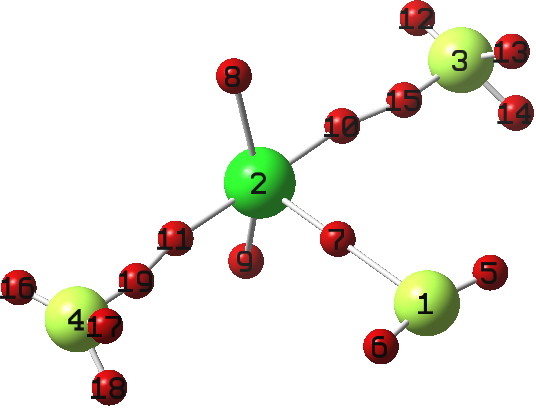
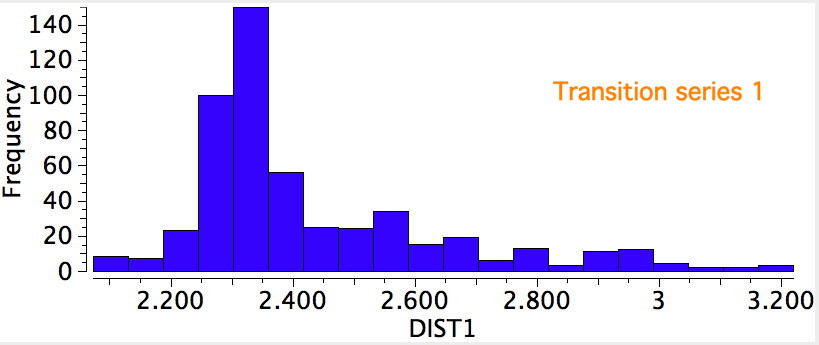
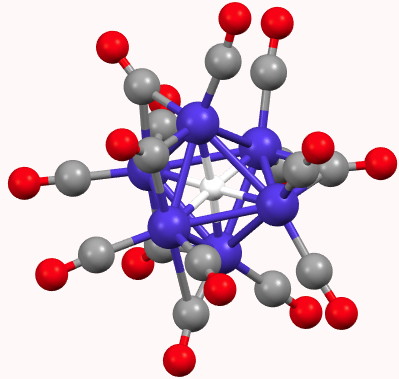
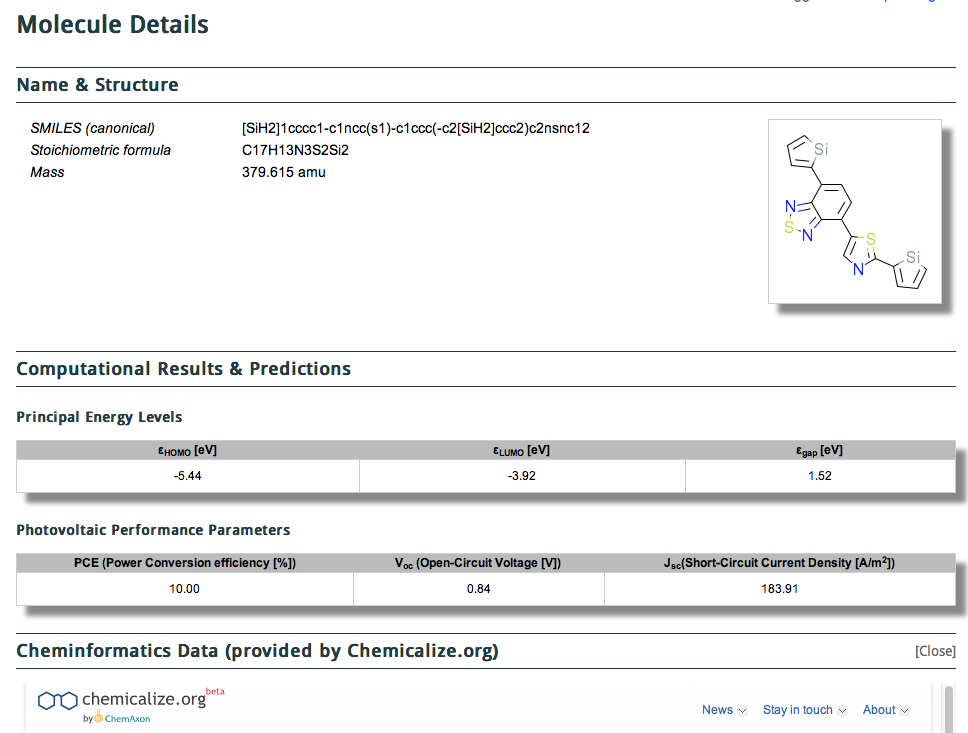
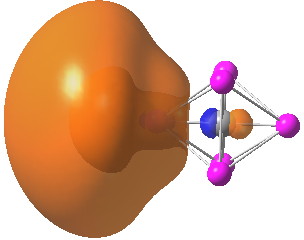
We have been experimenting with full-colour 3D printing of molecular objects. I thought I might here share some of our observations. Firstly, I list the software used: Crystal structures as sources of ball&stick models ( e.g. the CCDC database). Gaussian style cube files for sources of wavefunctions.