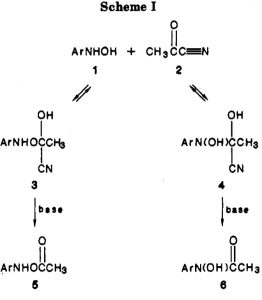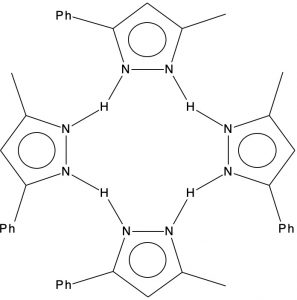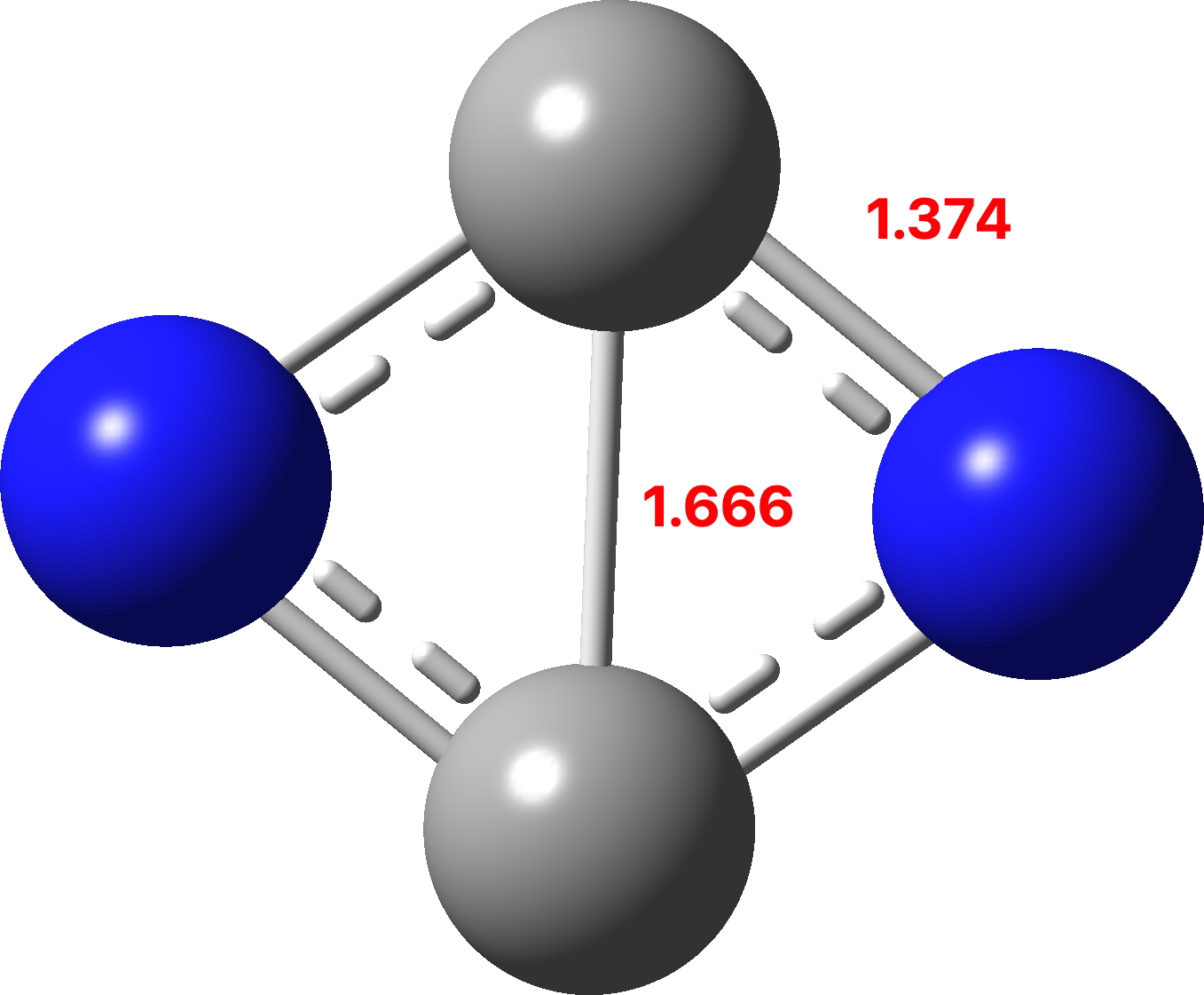
In 2006 we published an article illustrating various types of pseudorotations in small molecules. It’s been cited 20 times since then, so reasonable interest! We described rotations known as Lever and Turnstile as well as the better known Berry mode.