In 2011, I suggested that the standard monolith that is the conventional scientific article could be broken down into two separate, but interlinked components, being the story or narrative of the article and the data on which the story is based.

In 2011, I suggested that the standard monolith that is the conventional scientific article could be broken down into two separate, but interlinked components, being the story or narrative of the article and the data on which the story is based.
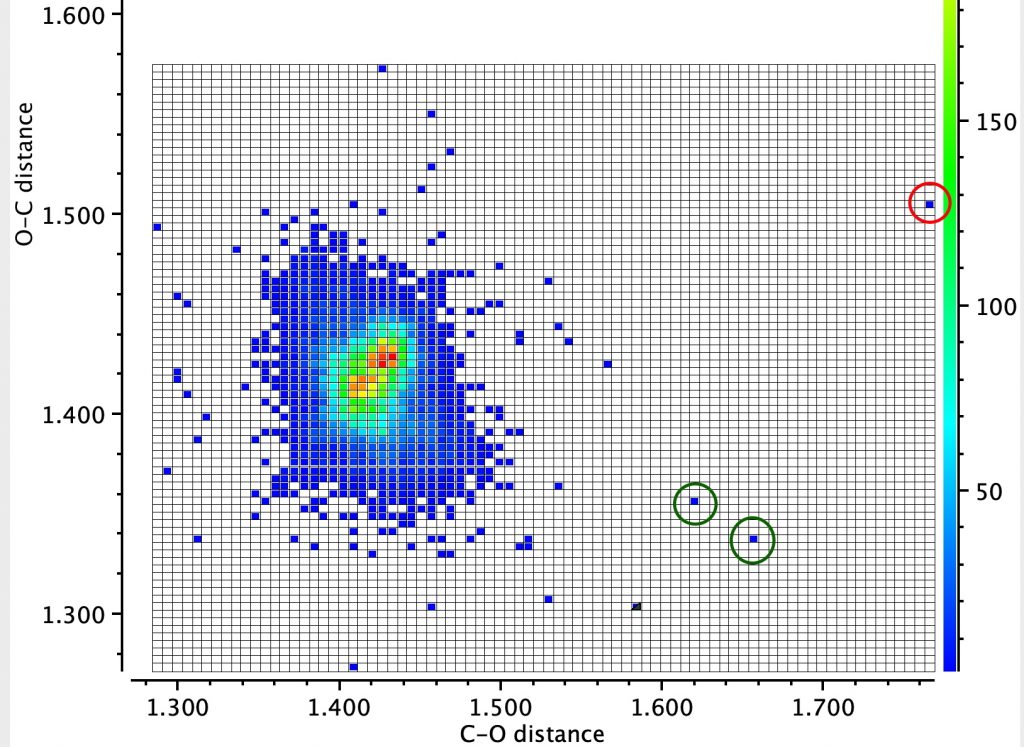
In the earlier post on the topic of anomeric effects, I identified a number of outliers associated with large differences in the lengths of two carbon-oxygen bonds sharing a common carbon atom.
In another post, a discussion arose about whether it might be possible to trap cyclopropenylidene to form a small molecule with a large dipole moment. Doing so assumes that cyclopropenylidene has a sufficiently long lifetime to so react, before it does so with itself to e.g. dimerise.
Occasionally, someone comments about an old post here, asking a question. Such was the case here, when a question about the dipole moment of cyclopropenylidene arose. It turned out to be 3.5D, but this question sparked a thought about the related molecule below.
The classic anomeric effect operates across a carbon atom attached to oxygens. One (of the two) lone pairs on the oxygen can donate into the σ* orbital of the C-O of the other oxygen (e.g. the red arrows) tending to weaken that bond whilst strengthening the donor oxygen C-O bond.
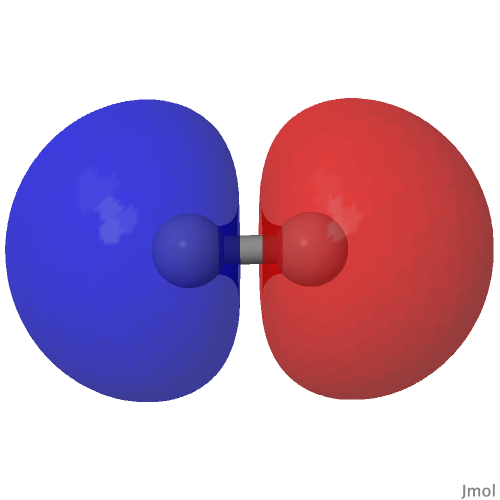
From the last few posts here, you might have noticed much discussion about how the element carbon might sustain a quadruple bond.
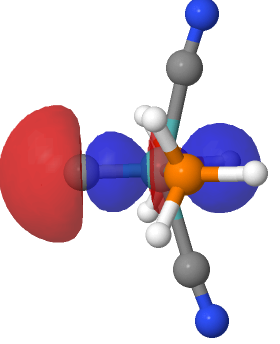
I noted in an earlier post the hypothesized example of (CO)3Fe⩸C as exhibiting a carbon to iron quadruple bond and which might have precedent in known five-coordinate metal complexes where one of the ligands is a “carbide” or C ligand.
The proposed identification of molecules with potential metal to carbon quadruple bonds, in which the metal exhibits trigonal bipyramidal coordination rather than the tetrahedral modes which have been proposed in the literature,, leads on to asking whether simple trigonal coordination at the metal can also sustain this theme?
Introductory chemistry will tell us that a triple bond between say two carbon atoms comprises just one bond of σ-axial symmetry and two of π-symmetry. Increasingly mentioned nowadays is the possibility of a quadruple bond between carbon and either itself or a transition metal, as discussed in the previous post.
Following from much discussion over the last decade about the nature of C2, a diatomic molecule which some have suggested sustains a quadruple bond between the two carbon atoms, new ideas are now appearing for molecules in which such a bond may also exist between carbon and a transition metal atom.
In the preceding post, I looked at a computed mechanism for the hydrolysis of a ketal by water.