A celebration of the life and work of the great chemist Paul von R. Schleyer was held this week in Erlangen, Germany. There were many fantastic talks given by some great chemists describing fascinating chemistry. Here I highlight the presentation given by Andy Streitwieser on the topic of organolithium chemistry, also a great interest of Schleyer's over the years.
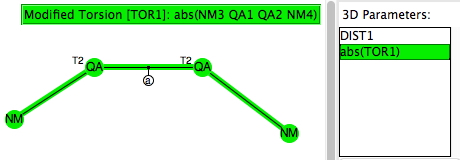
The upcoming ACS national meeting in San Diego has a CHED (chemical education division) session entitled Implementing Discovery-Based Research Experiences in Undergraduate Chemistry Courses. I had previously explored what I called extreme gauche effects in the molecule F-S-S-F. Here I take this a bit further to see what else can be discovered about molecules containing bonds between group 16 elements (QA= O, S, Se, Te).
At the precise moment I write this, there is information about 108,230,950 organic and inorganic chemical substances from the World's disclosed chemistry.
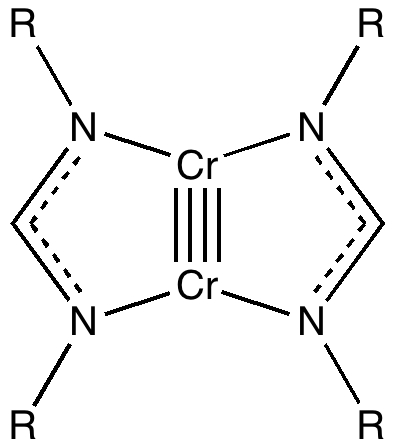
Six years ago, I posted on the nature of a then recently reported[cite]10.1002/anie.200803859[/cite] Cr-Cr quintuple bond. The topic resurfaced as part of the discussion on a more recent post on NSF 3 , and a sub-topic on the nature of the higher order bonding in C 2 . The comment made a connection between that discussion and the Cr-Cr bond alluded to above.
Earlier I explored models for the heteroaromatic electrophilic protiodecarboxylation of an 3-substituted indole, focusing on the role of water as the proton transfer and delivery agent. Next, came models for both water and the general base catalysed ionization of indolinones.
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then.
You might have noticed the occasional reference here to the upcoming centenary of the publication of Gilbert N. Lewis’ famous article entitled “ The atom and the molecule ”.[cite]10.1021/ja02261a002[/cite] A symposium exploring his scientific impact and legacy will be held in London on March 23, 2016, exactly 70 years to the day since his death. A list of the speakers and their titles is shown below;
The layout of floor 2 of the chemistry department here contains a number of small rooms which function as tutorial areas. Each has a (non-interactive) whiteboard used by students and tutors for, inter-alia , thought-showering. It was in one such room that I found myself with three colleagues this monday afternoon. We soon all sensed something not quite right about the room;
In answering tutorial problems, students often need skills in deciding how much time to spend on explaining what does not happen, as well as what does. Here I explore alternatives to the mechanism outlined in the previous post to see what computation has to say about what does (or might) not happen.
This reaction emerged a few years ago (thanks Alan!) as a tutorial problem in organic chemistry, in which students had to devise a mechanism for the reaction and use this to predict the stereochemical outcome at the two chiral centres indicated with *. It originates in a brief report from R. B. Woodward’s group in 1973 describing a prostaglandin synthesis,[cite]10.1021/ja00801a066[/cite] the stereochemical outcome being crucial.
