Metaldehyde is an insecticide used to control slugs.
With the current global lockdown, and students along with everyone else staying at home, I have noticed some old posts of mine are getting more attention than normal. One of these is an analysis I did in 2012 of Robinson’s original curly arrow illustration.
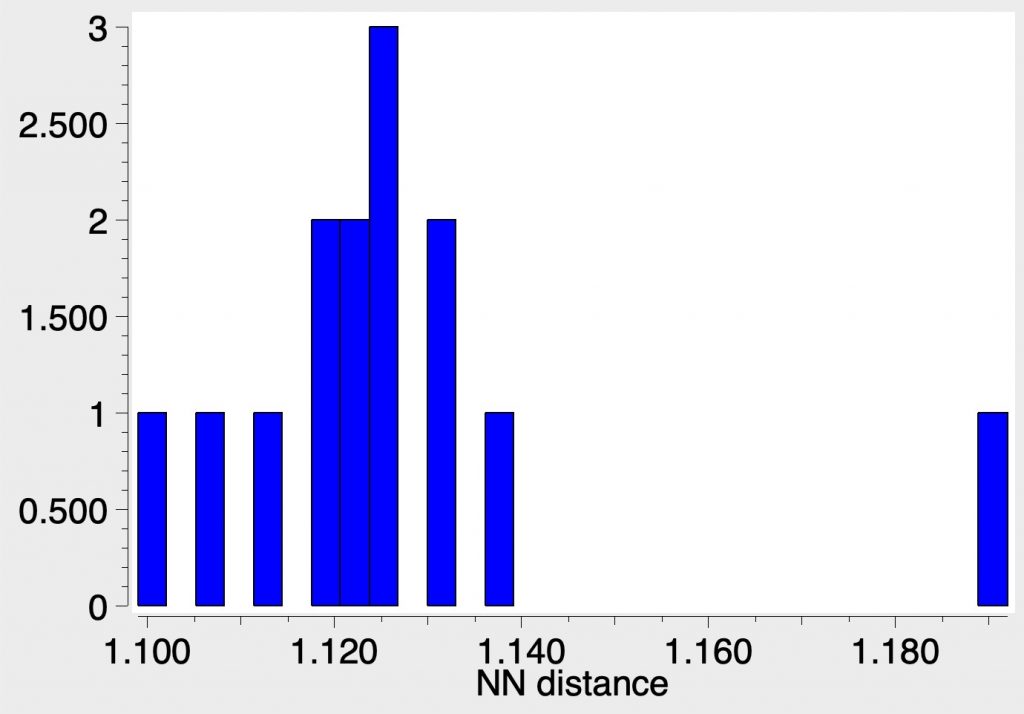
My previous two posts on the topic of strongest bonds have involved mono and diprotonating N2 and using quantum mechanics to predict the effect this has on the N-N bond via its length and vibrational stetching mode. Such species are very unlikely to be easily observed for verification.
I occasionally notice that posts that first appeared here many years ago suddenly attract attention.
Earlier, I explored the choreography or “timing”, of what might be described as the curly arrows for a typical taught reaction mechanism, the 1,4-addition of a nucleophile to an unsaturated carbonyl compound (scheme 1). I am now going to explore the consequences of changing one of the actors by adding the nucleophile to an unsaturated […]
A little more than a year ago, a ChemRxiv pre-print appeared bearing the title referenced in this post, which immediately piqued my curiosity. The report presented persuasive evidence, in the form of trapping experiments, that dicarbon or C2 had been formed by the following chemical synthesis.
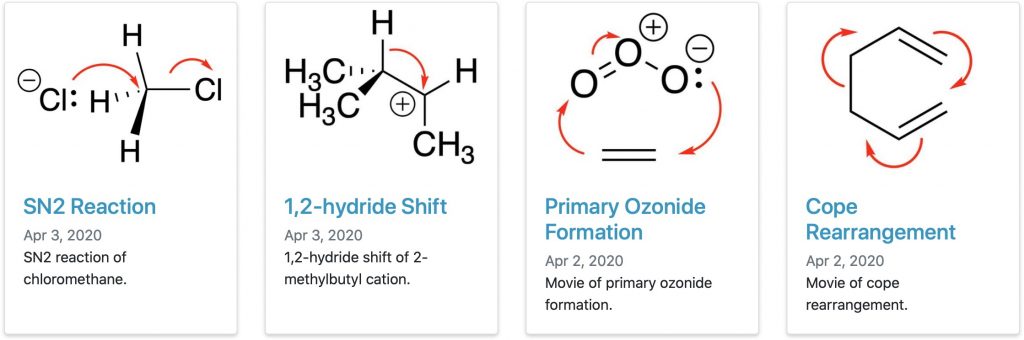
In a previous post, I talked about a library of reaction pathway intrinsic reaction coordinates (IRCs) containing 115 examples of organic and organometallic reactions.
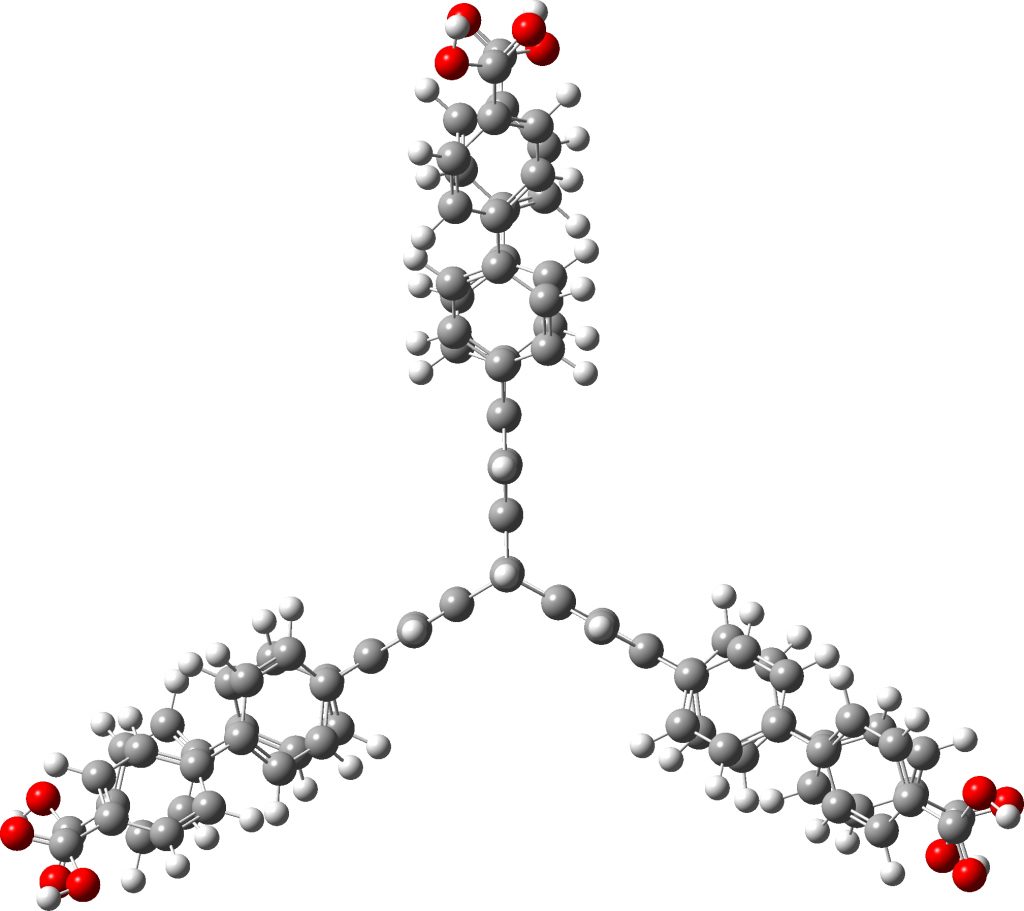
In the news this week is a report of a molecule whose crystal lattice is capable of both storing and releasing large amounts of hydrogen gas at modest pressures and temperatures. Thus “NU-1501-Al” can absorb 14 weight% of hydrogen.
A reaction can be thought of as molecular dancers performing moves. A choreographer is needed to organise the performance into the ballet that is a reaction mechanism. Here I explore another facet of the Michael addition of a nucleophile to a conjugated carbonyl compound.
In the previous post, I introduced three of a new generation of search engines specialising in the discovery of data.
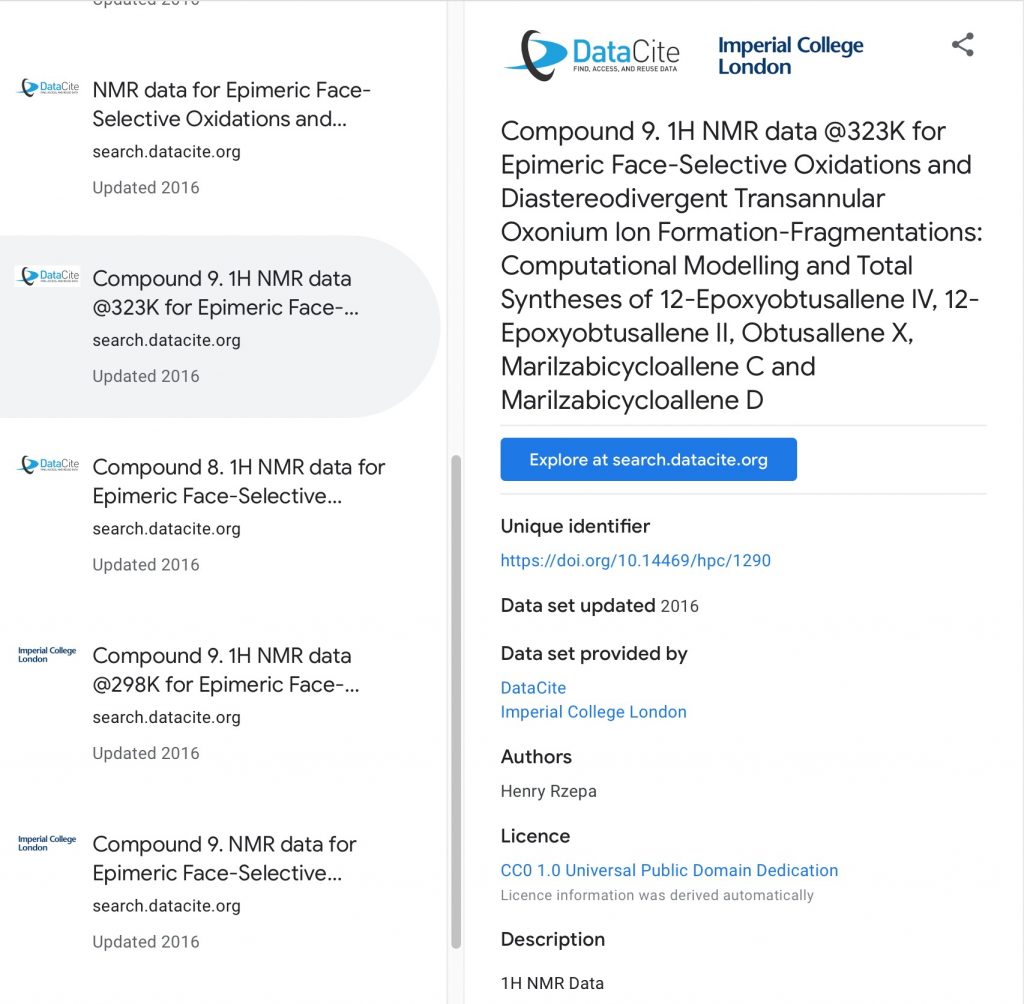
Chemists have long been familiar with search engines that aspire to index a large proportion of the chemical literature.