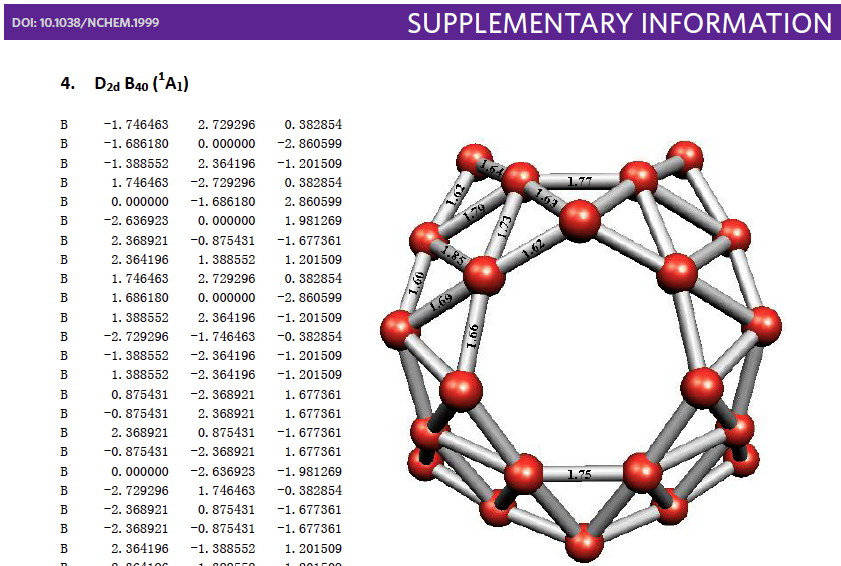
Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom ( X , acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A . They are superficially surprising, since both D and X look like electron rich species.