Around 100 tons of the potent antimalarial artemisinin is produced annually; a remarkable quantity given its very unusual and fragile looking molecular structure (below). When I looked at this, I was immediately struck by a thought: surely this is a classic molecule for analyzing stereoelectronic effects (anomeric and gauche). Here this aspect is explored.
Science is rarely about a totally new observation or rationalisation, it is much more about making connections between known facts, and perhaps using these connections to extrapolate to new areas (building on the shoulders of giants, etc). So here I chart one example of such connectivity over a period of six years.
My previous post related to the aromatic electrophilic substitution of benzene using as electrophile phenyl diazonium chloride. Another prototypical reaction, and again one where benzene is too inactive for the reaction to occur easily, is the catalyst-free bromination of benzene to give bromobenzene and HBr.
This is the time of year when I deliver two back-2-back lecture courses, and yes I do update and revise the content! I am always on the look-out for nice new examples that illustrate how concepts and patterns in chemistry can be joined up to tell a good story. My attention is currently on conformational analysis;
A game one can play with pericyclic reactions is to ask students to identify what type a given example is. So take for example the reaction below. The alternatives are: A cyclo-elimination reaction (red arrows). Two concurrent electrocyclic ring openings (blue and magenta arrows) Two consecutive electrocyclic ring openings Or could it be a hybrid with characteristics of both the first two?
Text books will announce that during aromatic electrophilic substitution, aromaticity is lost by the formation of a Wheland intermediate (and regained by eliminating a proton). Is that entirely true?
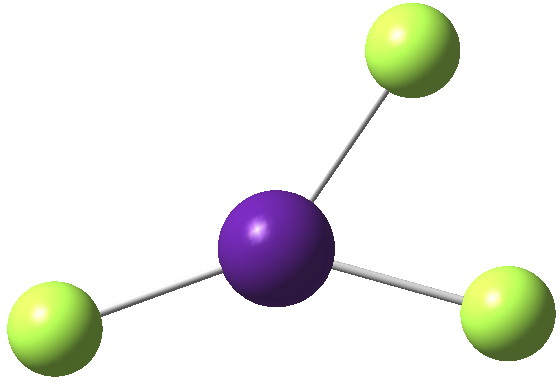
Mercury (IV) tetrafluoride attracted much interest when it was reported in 2007[cite]10.1002%2Fanie.200703710[/cite] as the first instance of the metal being induced to act as a proper transition element (utilising d-electrons for bonding) rather than a post-transition main group metal (utilising just s-electrons) for which the HgF 2 dihalide would be more normal (“Is mercury now a transition
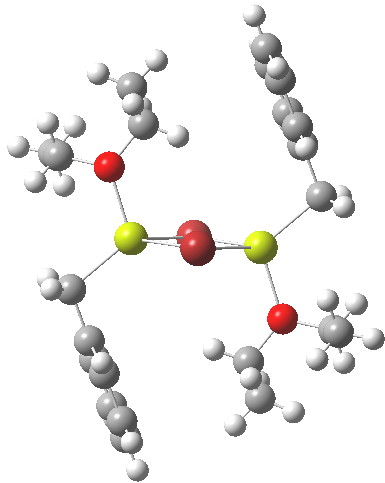
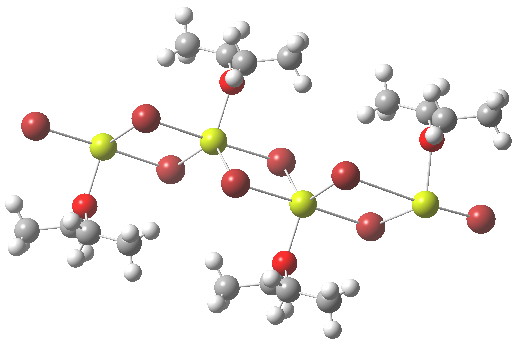
In the previous post I mentioned in passing the Grignard reagent benzyl magnesium bromide as having tetrahedral coordination at Mg. But I have now noticed, largely through spotting Steve Bachrach’s post on “Acene dimers – open or closed?” another geometric effect perhaps worthy of note, certainly one not always noted in the past; that of dispersion forces.
The following is a short question in a problem sheet associated with introductory organic chemistry.
The element silicon best represents the digital era of the mid 20th century to the present;
