White City is a small area in west london created as an exhibition site in 1908, morphing over the years into an Olympic games venue, a greyhound track, the home nearby of the BBC (British Broadcasting Corporation) and most recently the new western campus for Imperial College London.♣ The first Imperial department to move into […]
Harnessing FAIR data is an event being held in London on September 3rd; no doubt all the speakers will espouse its virtues and speculate about how to realize its potential.♥ Admirable aspirations indeed. Capturing hearts and minds also needs lots of real life applications!
Consider the four reactions.
FAIR is one of those acronyms that spreads rapidly, acquires a life of its own and can mean many things to different groups. A two-day event has just been held in Amsterdam to bring some of those groups from the chemical sciences together to better understand FAIR.

This last month, as a follow-up to the preceding post on the colour of flowers, I have been moonlighting by blogging elsewhere. Do go visit my “guerrilla blog” at perivalepark.london. Part of this project involves visiting two “physic or botanic” gardens, which originate from early 17th century explorations of herbs and other botanicals as medicines.
It was about a year ago that I came across a profusion of colour in my local Park. Although colour in fact was the topic that sparked my interest in chemistry many years ago (the fantastic reds produced by diazocoupling reactions), I had never really tracked down the origin of colours in many flowers.
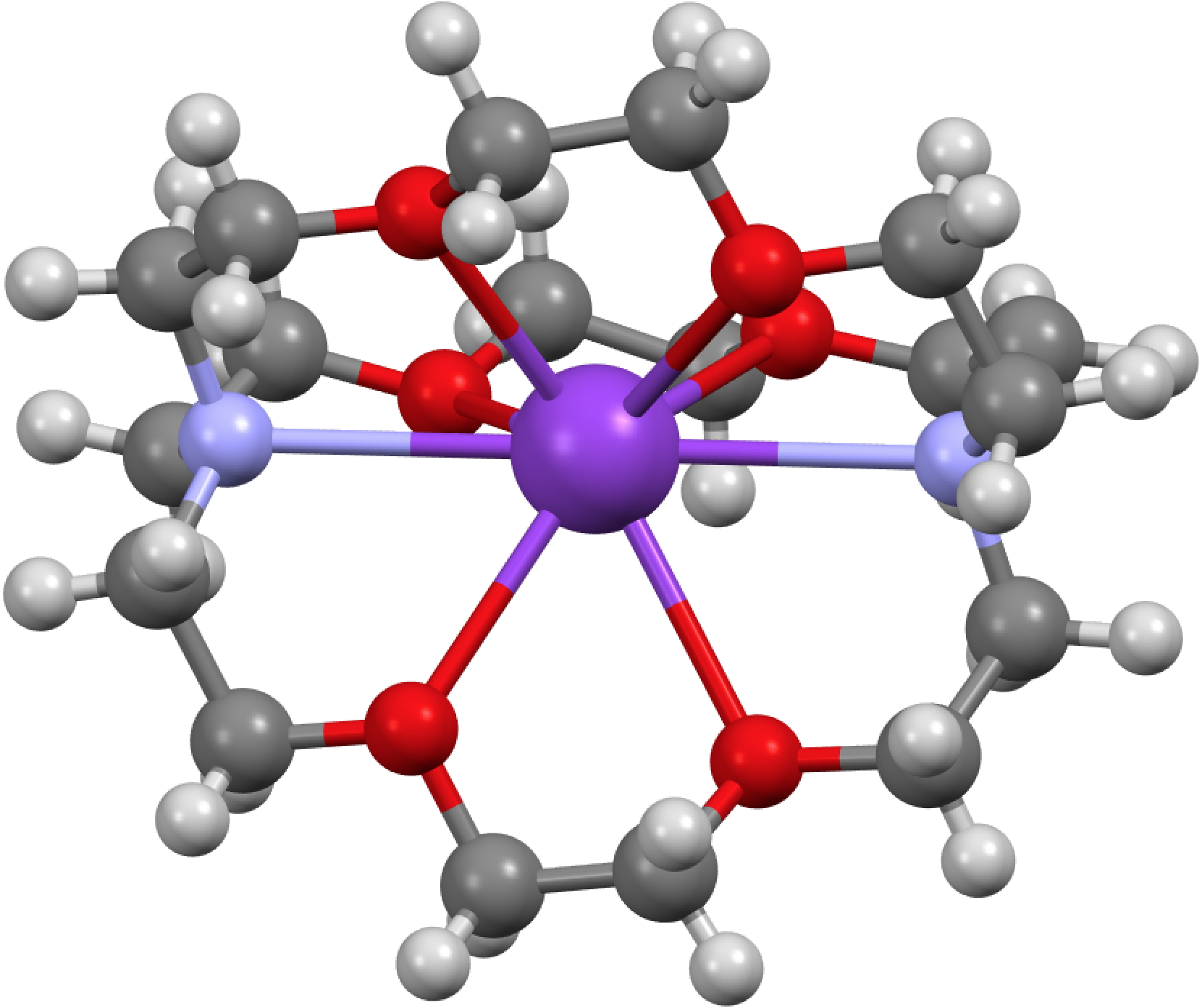
Ten years are a long time when it comes to (recent) technologies. The first post on this blog was on the topic of how to present chemistry with three intact dimensions.
The site fairsharing.org is a repository of information about FAIR (Findable, Accessible, Interoperable and Reusable) objects such as research data.
The molecules below were discussed in the previous post as examples of highly polar but formally neutral molecules, a property induced by aromatisation of up to three rings.
In several posts a year or so ago I considered various suggestions for the most polar neutral molecules, as measured by the dipole moment. A record had been claimed for a synthesized molecule of ~14.1±0.7D. I pushed this to a calculated 21.7D for an admittedly hypothetical and unsynthesized molecule.

Around the time of the 2012 olympic games, the main site for which was Stratford in east London, I heard a fascinating talk about the “remediation” of the site from the pollution caused by its industrial chemical heritage. Here I visit another, arguably much more famous and indeed older industrial site.