
The triennial conference is this year located in Munich. With 1500 participants and six parallel sessions, this report can give only a flavour of proceedings.

The triennial conference is this year located in Munich. With 1500 participants and six parallel sessions, this report can give only a flavour of proceedings.
At the moment, the bond slam is something of a home from home for this blog and since much of my activity is happening there rather than here, I thought I might give you pointers to some of the topics, which are evolving, so to speak, before our very eyes.
It is always interesting to observe conference experiments taking place. The traditional model involves travelling to a remote venue, staying in a hotel, selecting sessions to attend from a palette of parallel streams and then interweaving chatting to colleagues both old and new over coffee, lunch, dinner or excursions.

Bees are having a tough time around the world. Oddly, they are surviving very well in cities. One reason are the wild flower meadows in London and for some summer relief I thought I would tell you the story of the one shown below.
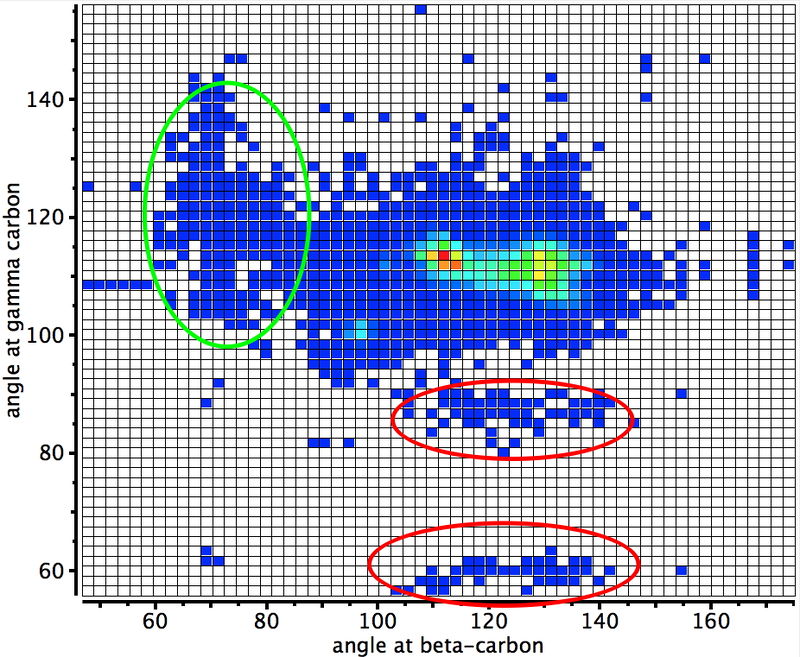
There is much focus at the moment on how to ensure experimental replicability in e.g. the molecular sciences. An important aspect of that is having access to FAIR data; data which is findable, accessible, inter-operable and re-usable. One of the “gold standards” in chemistry is the data associated with crystal structures.
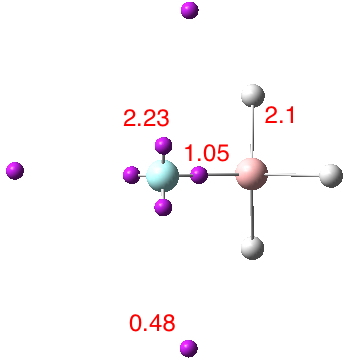
The effects of loading up lots of dispersion attractions (between t-butyl groups) into a compact molecule has the interesting consequence of allowing two “non-bonded” hydrogen atoms to approach to ~1.5Å of each other, thus creating the appearance of a “bond” where one normally would not be found.
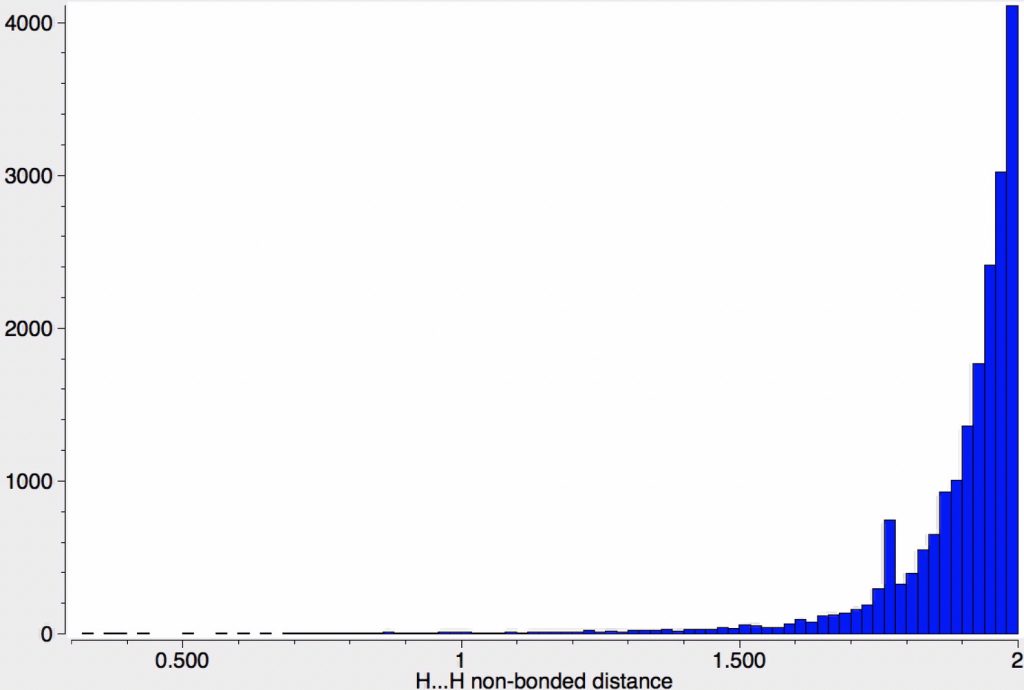
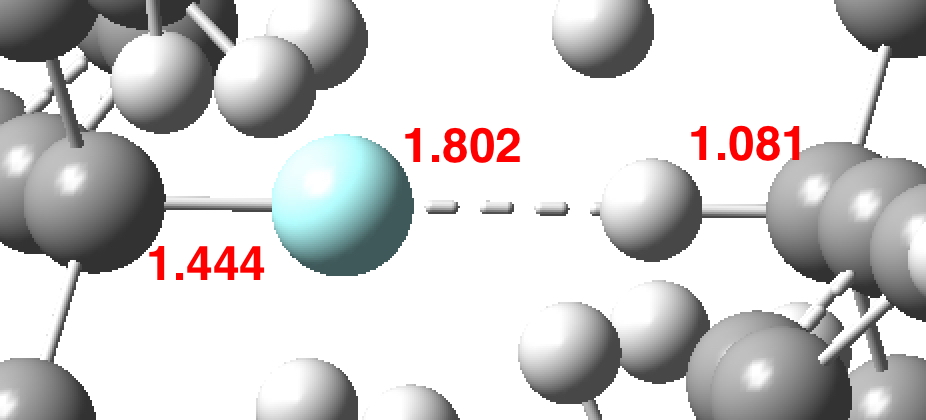
In the previous post, I noted the crystallographic detection of an unusually short non-bonded H…H contact of ~1.5Å, some 0.9Å shorter than twice the van der Waals radius of hydrogen (1.2Å, although some sources quote 1.1Å which would make the contraction ~0.7Å). This was attributed to dispersion attractions accumulating in the rest of the molecule.
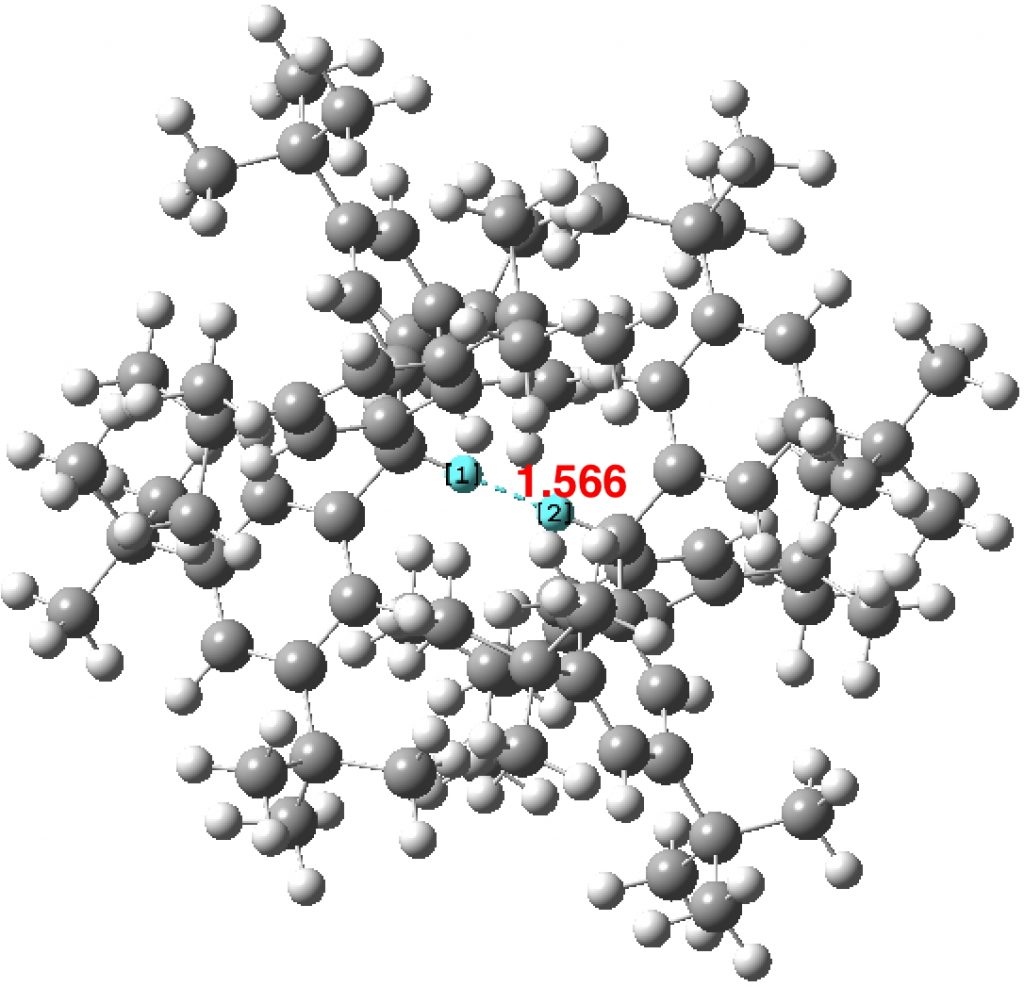
About 18 months ago, there was much discussion on this blog about a system reported by Bob Pascal and co-workers containing a short H…H contact of ~1.5Å. In this system, the hydrogens were both attached to Si as Si-H…H-Si and compressed together by rings.
The iron complex shown below forms the basis for many catalysts. With iron, the catalytic behaviour very much depends on the spin-state of the molecule, which for the below can be either high (hextet) or medium (quartet) spin, with a possibility also of a low spin (doublet) state.
In an earlier post, I lamented the modern difficulties in running old instances of Jmol, an example of an application program written in the Java programming language. When I wrote that, I had quite forgotten a treasure trove of links to old Java that I had collected in 1996-7 and then abandoned.
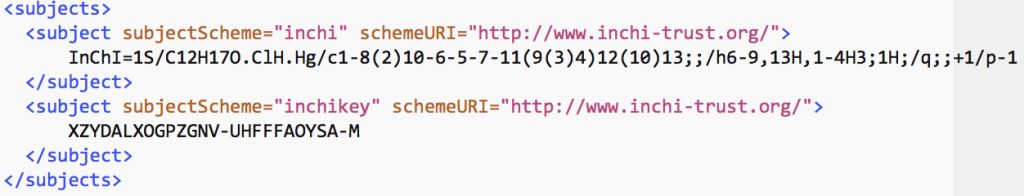
As data repositories start to flourish, it is reasonable to ask questions such as what sort of chemistry can be found there and how can I find it? Here I give an updated worked example of a digital repository search for chemical content and also pose an important issue for the chemistry domain.