The mechanism of forming an oxime from nucleophilic addition of a hydroxylamine to a ketone is taught early on in most courses of organic chemistry. Here I subject the first step of this reaction to form a tetrahedral intermediate to quantum mechanical scrutiny. The first decision is to decide which atom of the hydroxylamine acts as the nucleophile. Reaction 1 shows the oxygen and reaction 2 the nitrogen.
Semibullvalene is a molecule which undergoes a facile [3,3] sigmatropic shift. So facile that it appears this equilibrium can be frozen out at the transition state if suitable substituents are used. This is a six-electron process, which leads to one of those homologous questions; what happens with ten electrons? A 5,5 double Möbius sigmatropic rearrangement. Click for 3D model.
I am following up on one unfinished thread in my previous post; a candidate was proposed in which the transition state for [3,3] sigmatropic rearrangement in a semibullvalene might be frozen out to become instead a stable minimum. The hypothesis was that such a species would be aromatic, a bis -homoaromatic species to be precise, itself an interesting variation on the normal theme of aromaticity.
Semibullvalene is an unsettling molecule. Whilst it has a classical structure describable by a combination of Lewis-style two electron and four electron bonds, its NMR behaviour reveals it to be highly fluxional. This means that even at low temperatures, the position of these two-electron bonds rapidly shifts in the equilibrium shown below. Nevertheless, this dynamic behaviour can be frozen out at sufficiently low temperatures.
Here is a challenge: what is the longest C-C bond actually determined (in which both carbon termini are sp 3 hybridised)? I pose this question since Steve Bachrach has posted on how to stabilize long bonds by attractive dispersive interactions, and more recently commenting on what the longest straight chain alkane might be before dispersive interaction start to fold it (the answer appears to be C 17 ). A search of the
It was three years ago that I first blogged on the topic of the Sn2 reaction. Matthias Bickelhaupt had suggested that the Sn2 reaction involving displacement at a carbon atom was an anomaly; the true behaviour was in fact exhibited by the next element down in the series, silicon.
Recollect, Robinson was trying to explain why the nitroso group appears to be an o/p director of aromatic electrophilic substitution. Using σ/π orthogonality, I suggested that the (first ever) curly arrows as he drew them could not be the complete story, and that a transition state analysis would be needed. Here it is. Let me set the scene on how this might be done.
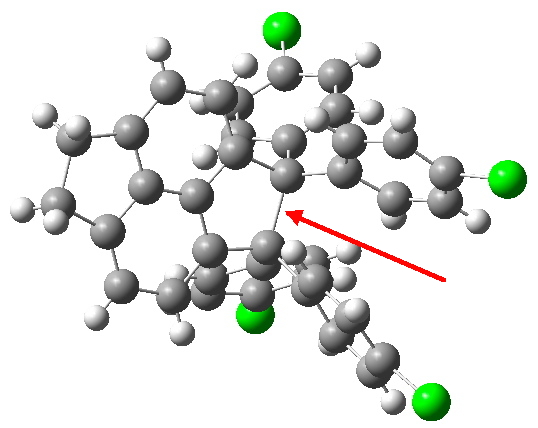
Singleton and co-workers have produced some wonderful work showing how dynamic effects and not just transition states can control the outcome of reactions. Steve Bachrach’s blog contains many examples, including this recent one. This shows that tolyl thiolate (X=Na) reacts with the dichlorobutenone to give two substitution products in a 81:19 ratio.
Years ago, I was travelling from Cambridge to London on a train. I found myself sitting next to a chemist, and (as chemists do), he scribbled the following on a piece of paper. When I got to work the next day Vera (my student) was unleashed on the problem, and our thoughts were published[cite]10.1039/C39920001323[/cite]. That was then. This is now.
Back in 1994, we published the crystal structure of the molecule below (X=H), a putative anti-malarial drug called halofantrine . Little did we realise that a whole area of organo catalysis based on a thiourea catalyst with a similar motif would emerge a little later. Here is how the two are connected. In our original article we described how our interest was sparked by observing the following chiral HPLC behaviour.
