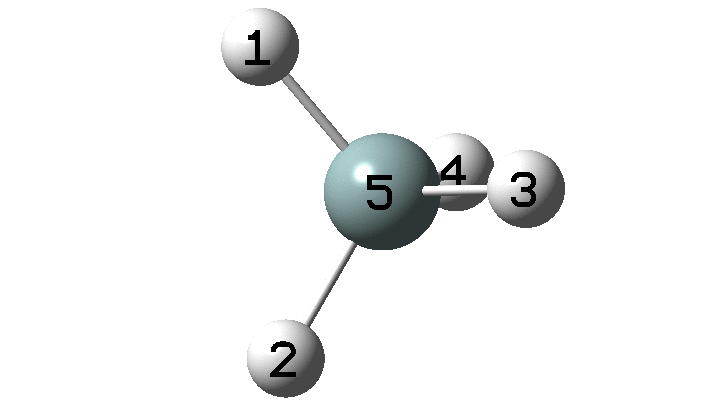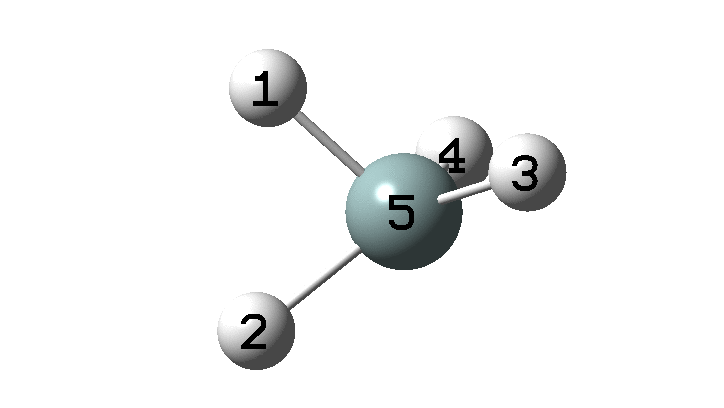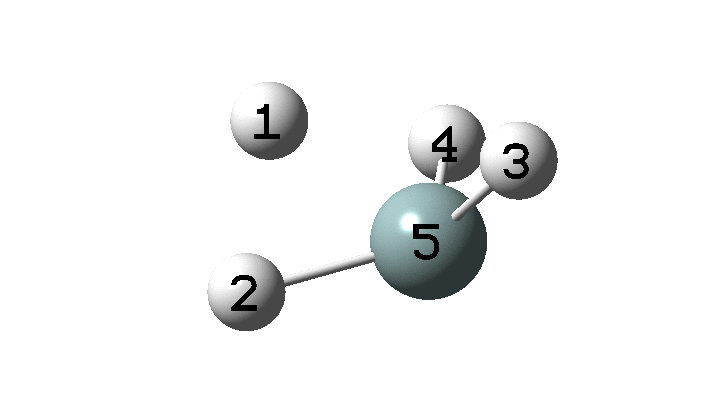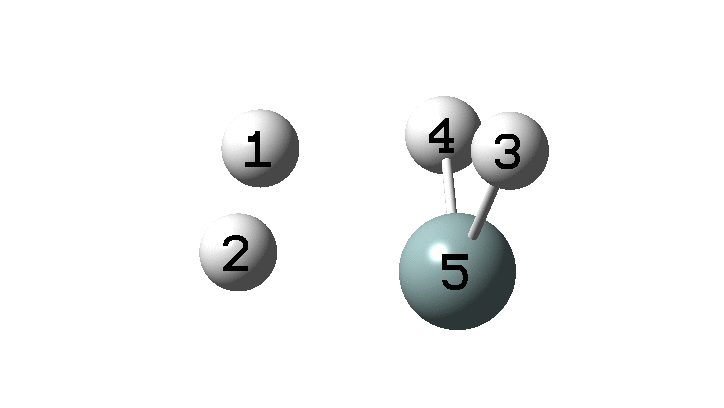
In the previous post, I found intriguing the mechanism by which methane (CH4) inverts by transposing two of its hydrogens. Here I take a look at silane, SiH4.

In the previous post, I found intriguing the mechanism by which methane (CH4) inverts by transposing two of its hydrogens. Here I take a look at silane, SiH4.
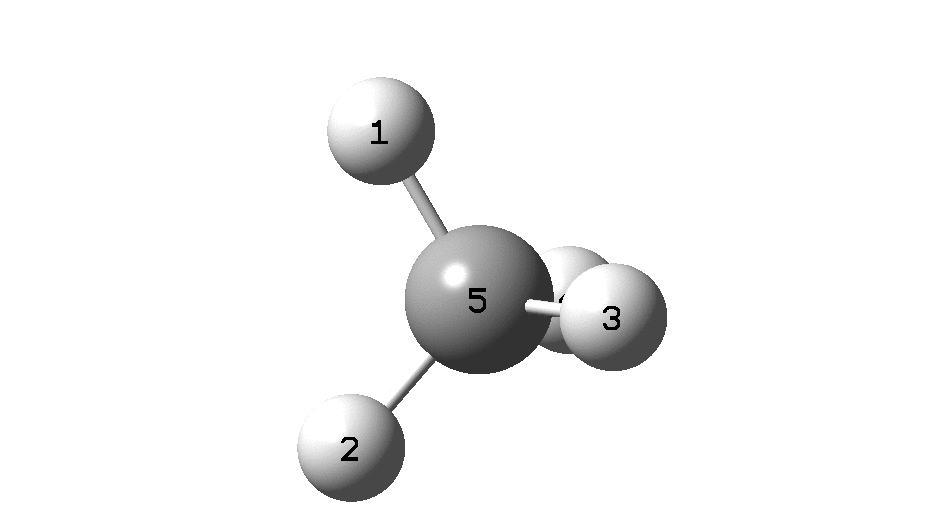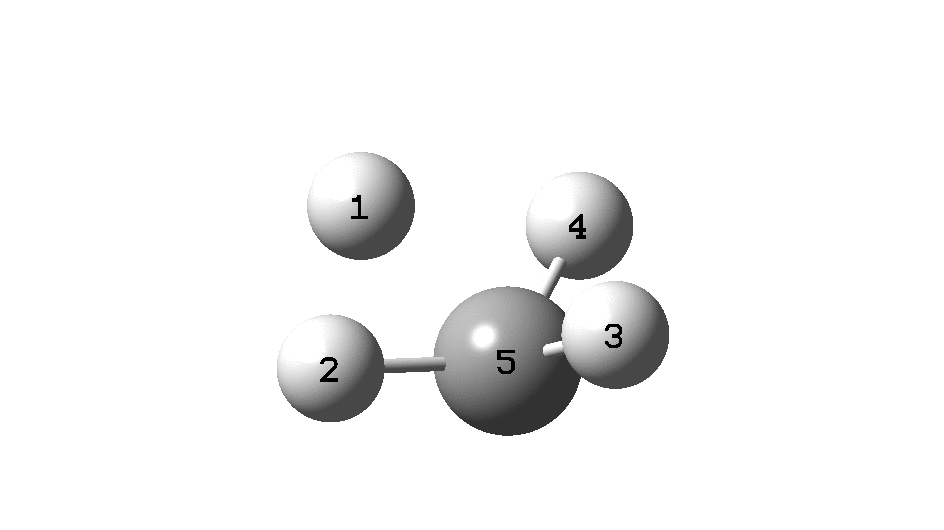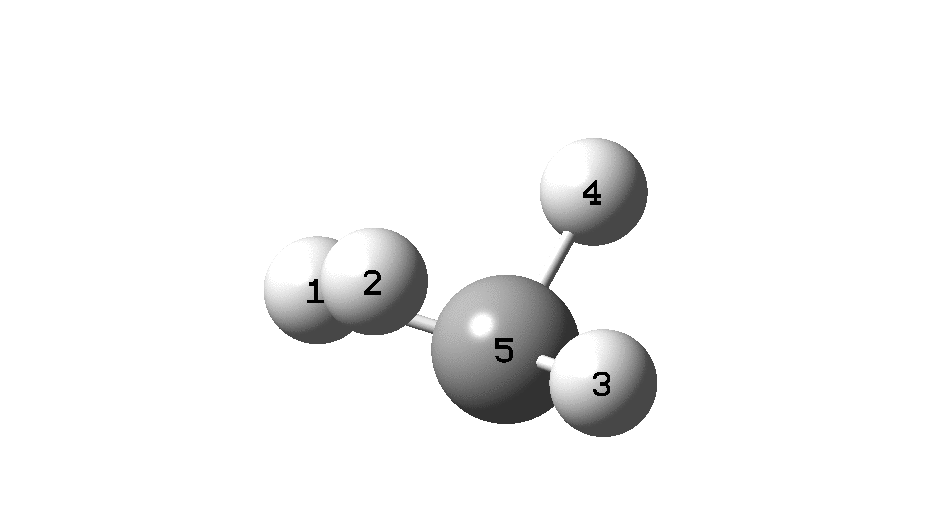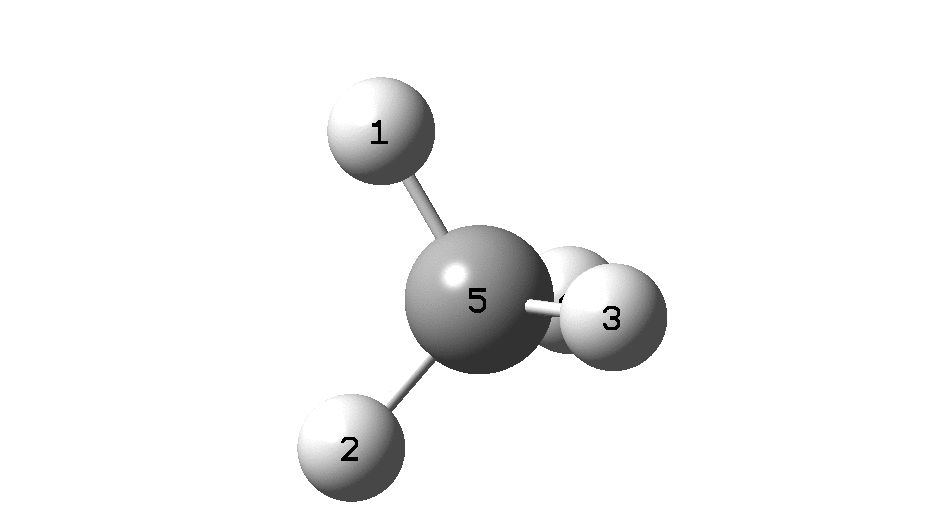
This is a spin-off from the table I constructed here for further chemical examples of the classical/non-classical norbornyl cation conundrum. One possible entry would include the transition state for inversion of methane via a square planar geometry as compared with e.g. NiH4 for which the square planar motif is its minimum.
This is another of those posts that has morphed from an earlier one noting the death of the great chemist George Olah.
George Olah passed away on March 8th. He was part of the generation of scientists in the post-war 1950s who had access to chemical instrumentation that truly revolutionised chemistry.
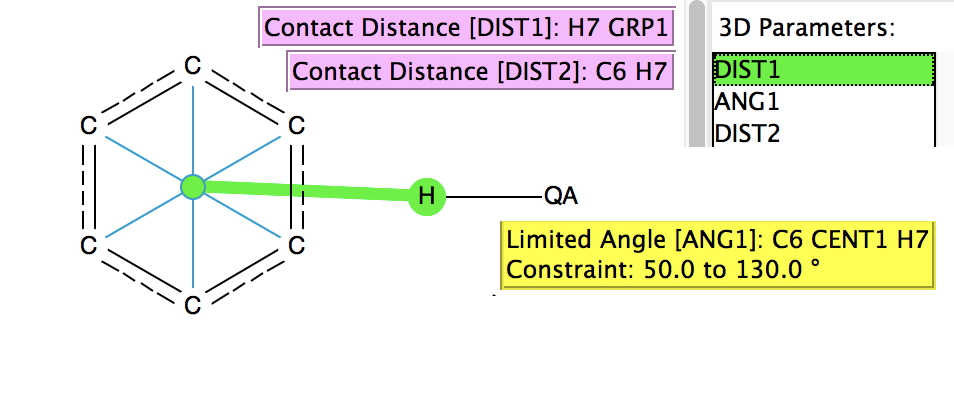
A few years back, I did a post about the Pirkle reagent and the unusual π-facial hydrogen bonding structure it exhibits. For the Pirkle reagent, this bonding manifests as a close contact between the acidic OH hydrogen and the edge of a phenyl ring;
Living in London, travelling using public transport is often the best way to get around. Before setting out on a journey one checks the status of the network. Doing so today I came across this page: our open data from Transport for London.

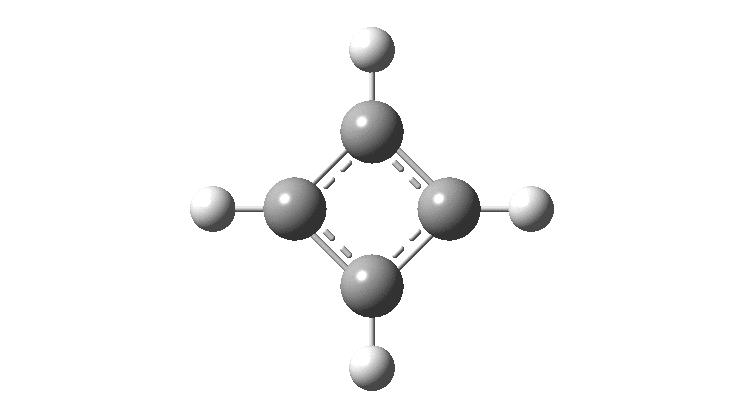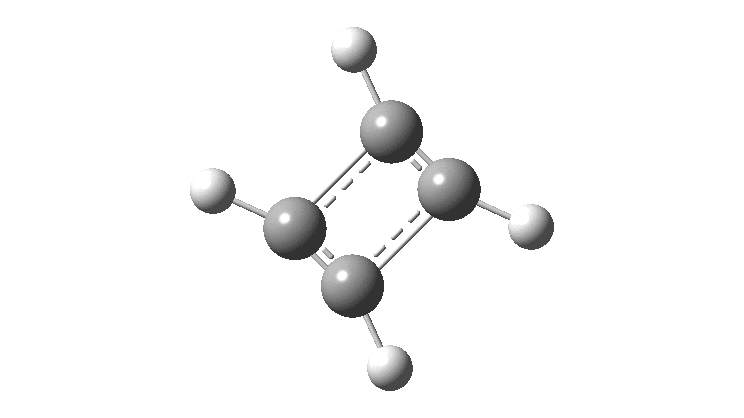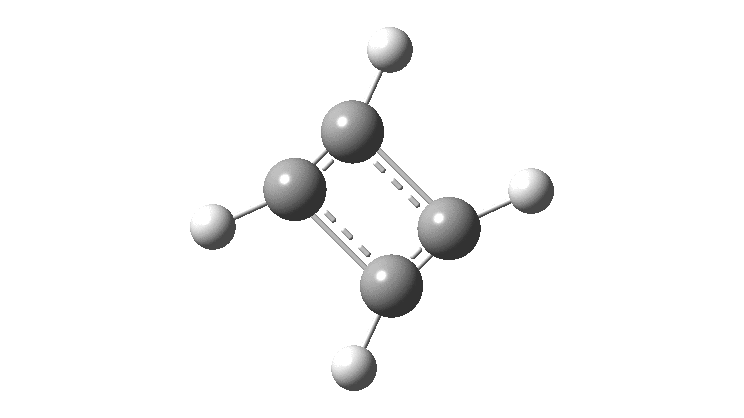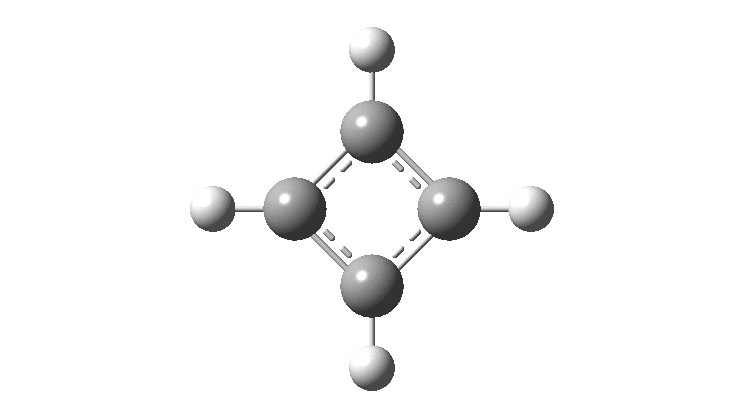
Cyclobutadiene is one of those small iconic molecules, the transience and instability of which was explained theoretically long before it was actually detected in 1965. Given that instability, I was intrigued as to how many crystal structures might have been reported for this ring system, along with the rather more stable congener cyclo-octatetraene.
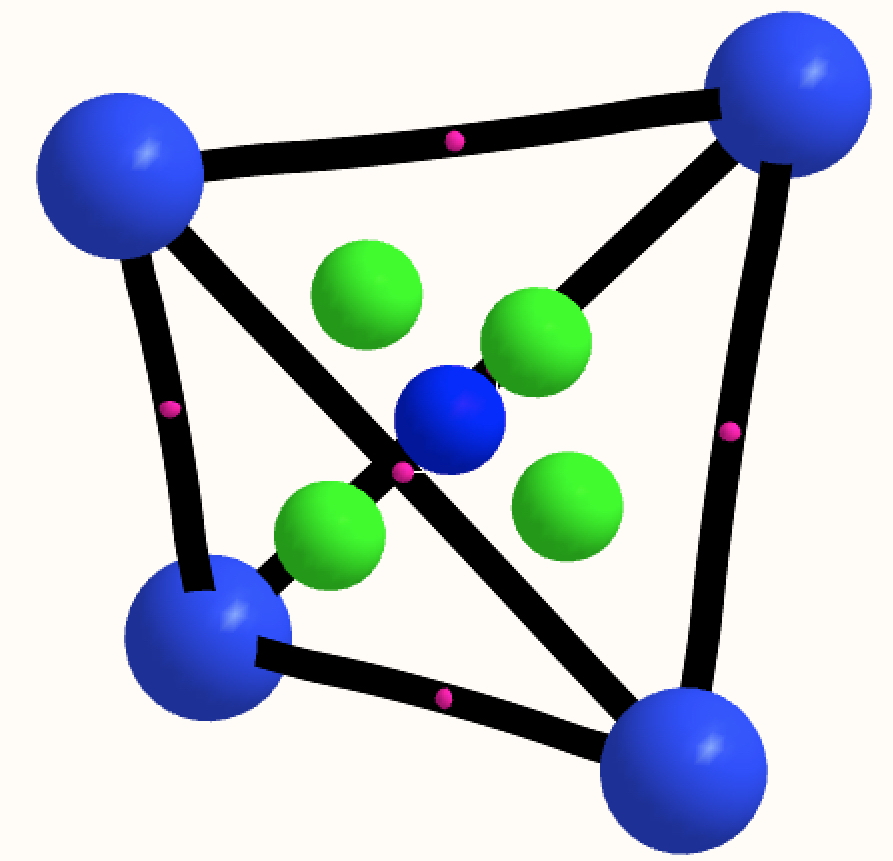
The thread thus far. The post about Na2He introduced the electride anionic counter-ion to Na+ as corresponding topologically to a rare feature known as a non-nuclear attractor.
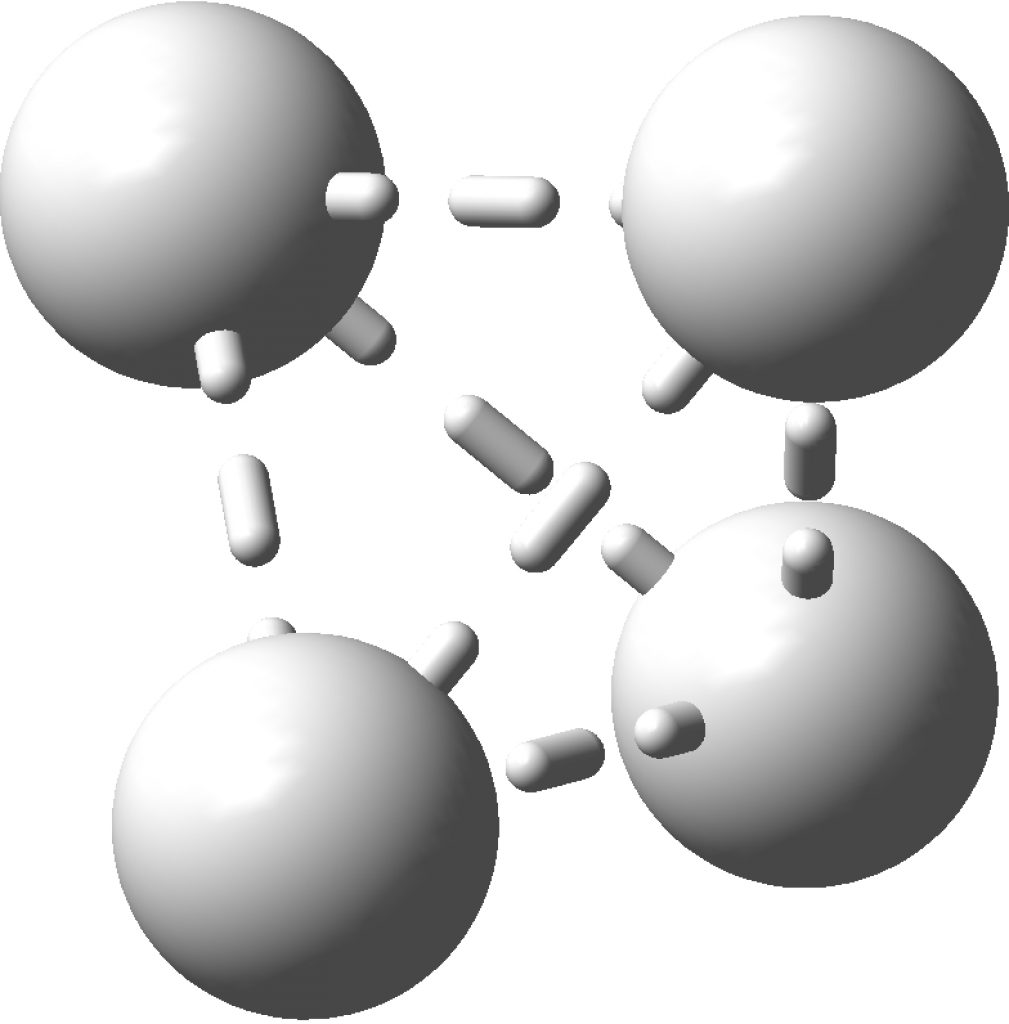
This post arose from a comment attached to the post on Na2He and relating to peculiar and rare topological features of the electron density in molecules called non-nuclear attractors. This set me thinking about other molecules that might exhibit this and one of these is shown below.

I analysed the bonding in chlorine trifluoride a few years back in terms of VSEPR theory. I noticed that several searches on this topic which led people to this post also included a query about the differences between it and the bromine analogue. For those who posed this question, here is an equivalent analysis.
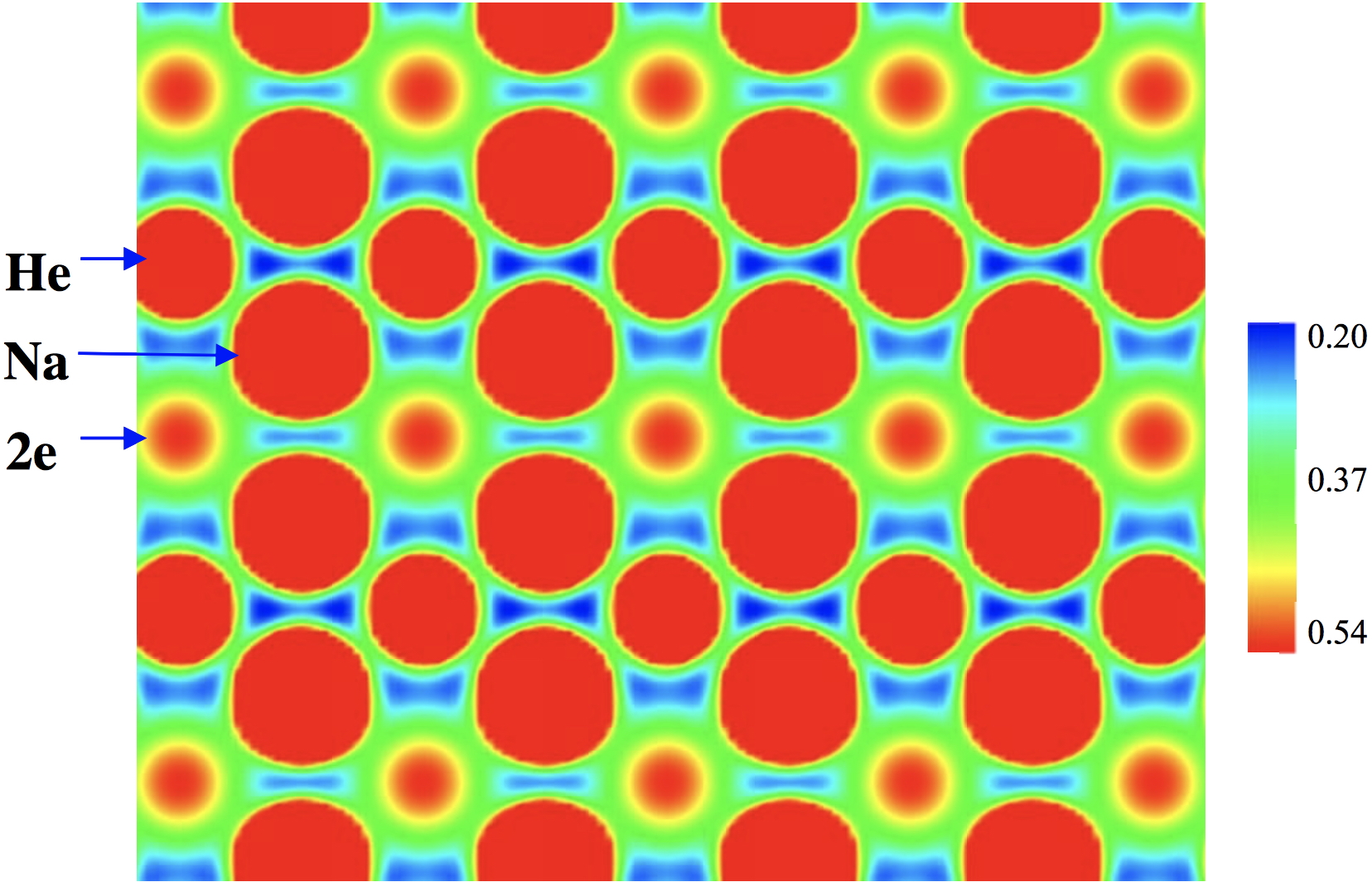
On February 6th I was alerted to this intriguing article by a phone call, made 55 minutes before the article embargo was due to be released. Gizmodo wanted to know if I could provide an (almost)† instant‡ quote.