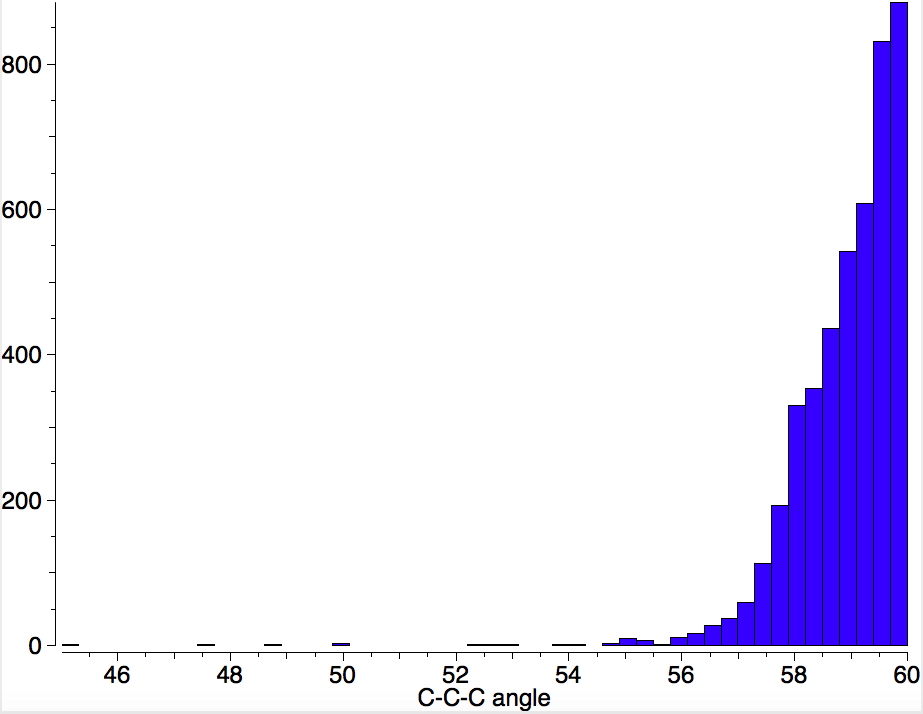
Is asking a question such as “what is the smallest angle subtended at a chain of three connected 4-coordinate carbon atoms” just seeking another chemical record, or could it unearth interesting chemistry?

Is asking a question such as “what is the smallest angle subtended at a chain of three connected 4-coordinate carbon atoms” just seeking another chemical record, or could it unearth interesting chemistry?
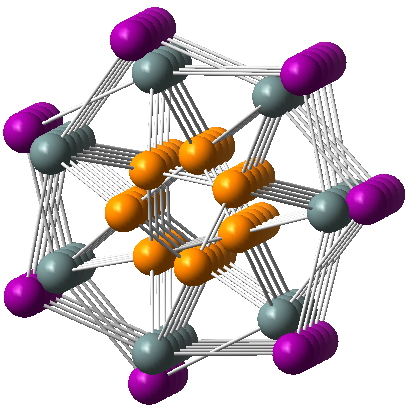
After sixty years of searching, the first non-templated double helical carbon-free inorganic molecular structure has been reported. That is so neat that I thought to load the 3D coordinates here for you to interact with and then to explore the prospect of using these coordinates to add some value with e.g. some chiroptical calculations.
Chemists are as fond of records as any, although I doubt you will find many chemical ones in the Guinness world records list. Polytriangulanes chase how many cyclopropyl 3-rings can be joined via a vertex.
Previously, a mechanistic twist to the oxidation of imines using peracid had emerged. Time to see how substituents respond to this mechanism.
Peter Murray-Rust and I are delighted to announce that the 2016 award of the Bradley-Mason prize for open chemistry goes to Jan Szopinski (UG) and Clyde Fare (PG).
The story so far. Imines react with a peracid to form either a nitrone (σ-nucleophile) or an oxaziridine (π-nucleophile). The balance between the two is on an experimental knife-edge, being strongly influenced by substituents on the imine.
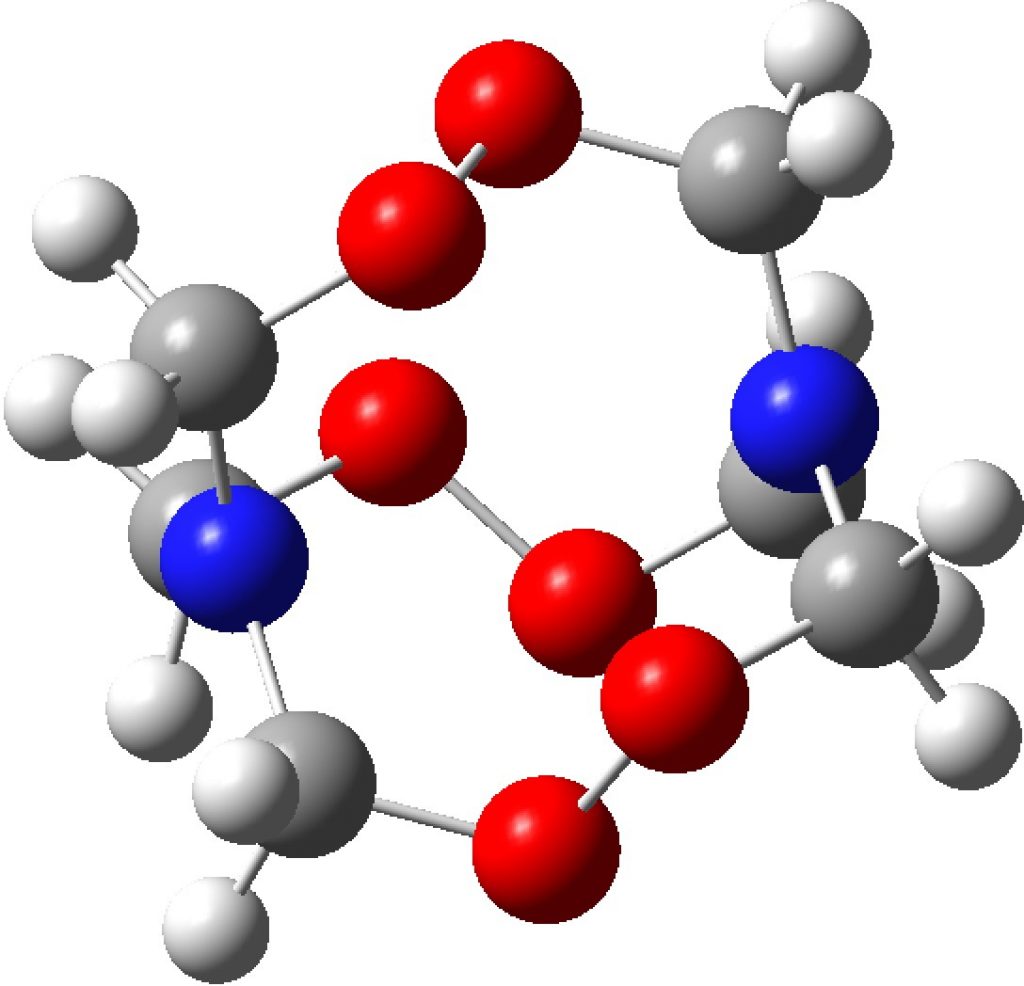
Compounds with O-O bonds often have weird properties. For example, artemisinin, which has some fascinating stereoelectronics. Here is another such, recently in the news and known as HMTD (hexamethylene triperoxide diamine). The crystal structure was reported some time ago and the article included an inspection of the computed wavefunction.
Nucleophiles are species that seek to react with an electron deficient centre by donating a lone or a π-bond pair of electrons. The ambident variety has two or more such possible sources in the same molecule, an example of which might be hydroxylamine or H2NOH.
I previously explored stabilized “carbenes” with the formal structures (R2N)2C:, concluding that perhaps the alternative ionic representation R2N+=C–NR2 might reflect their structures better.
To quote from Wikipedia: in chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The most ubiquitous type of carbene of recent times is the one shown below as 1, often referred to as a resonance stabilised or persistent carbene.
Bromoallene is a pretty simple molecule, with two non-equivalent double bonds. How might it react with an electrophile, say dimethyldioxirane (DMDO) to form an epoxide? Here I explore the difference between two different and very simple approaches to predicting its reactivity.