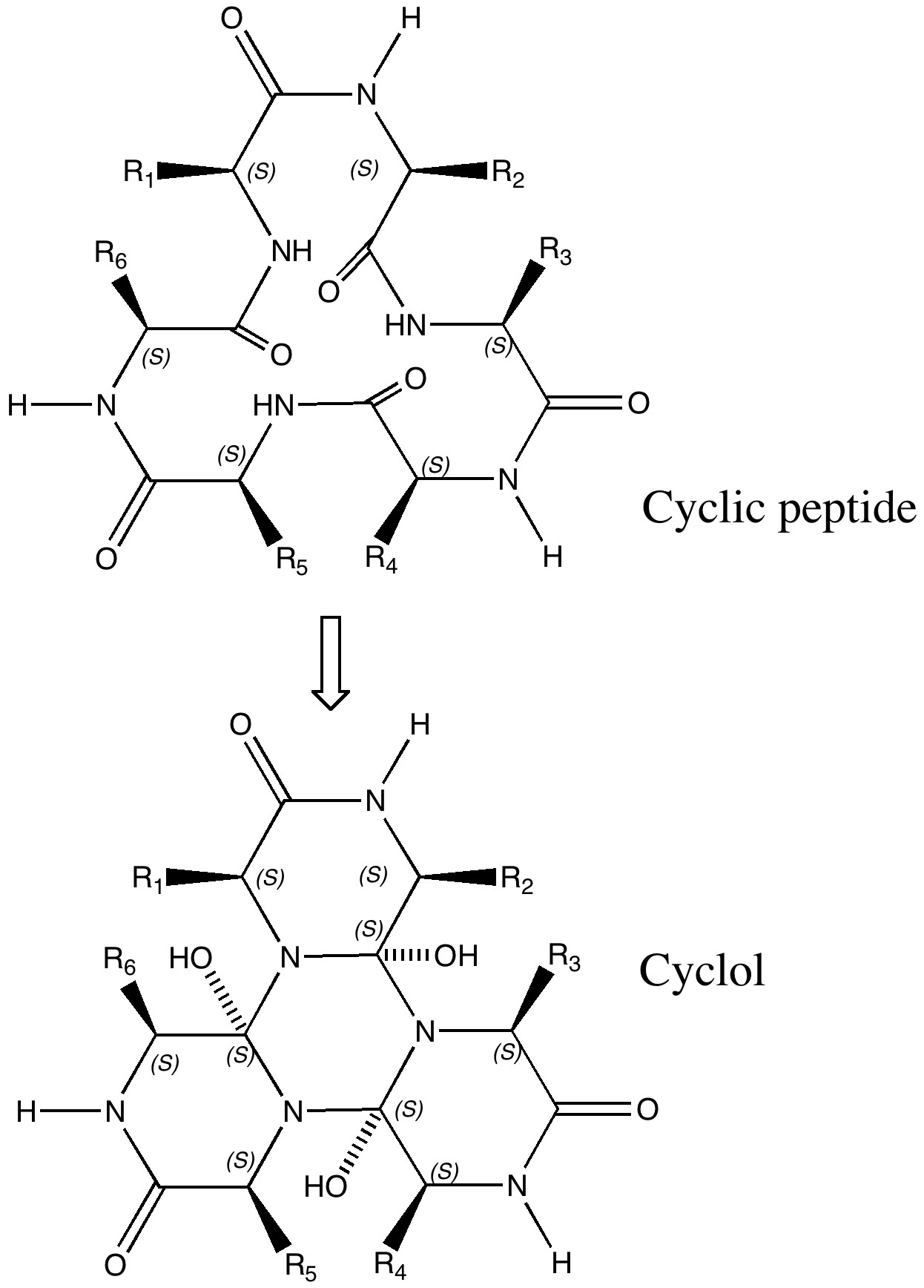
Most scientific theories emerge slowly, over decades, but others emerge fully formed virtually overnight as it were (think Einstein in 1905). A third category is the supernova type, burning brightly for a short while, but then vanishing (almost) without trace shortly thereafter.