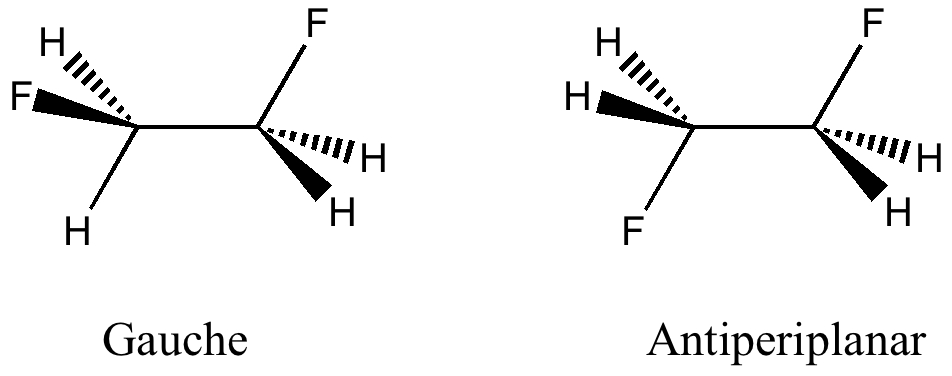
Here I offer another spin-off from writing a lecture course on conformational analysis. This is the famous example of why 1,2-difluoroethane adopts a gauche rather than antiperiplanar conformation.

Here I offer another spin-off from writing a lecture course on conformational analysis. This is the famous example of why 1,2-difluoroethane adopts a gauche rather than antiperiplanar conformation.
One of the (not a few) pleasures of working in a university is the occasional opportunity that arises to give a new lecture course to students.
One future vision for chemistry over the next 20 years or so is the concept of having machines into which one dials a molecule , and as if by magic, the required specimen is ejected some time later. This is in some ways an extrapolation of the existing peptide and nucleotide synthesizer technologies and sciences.
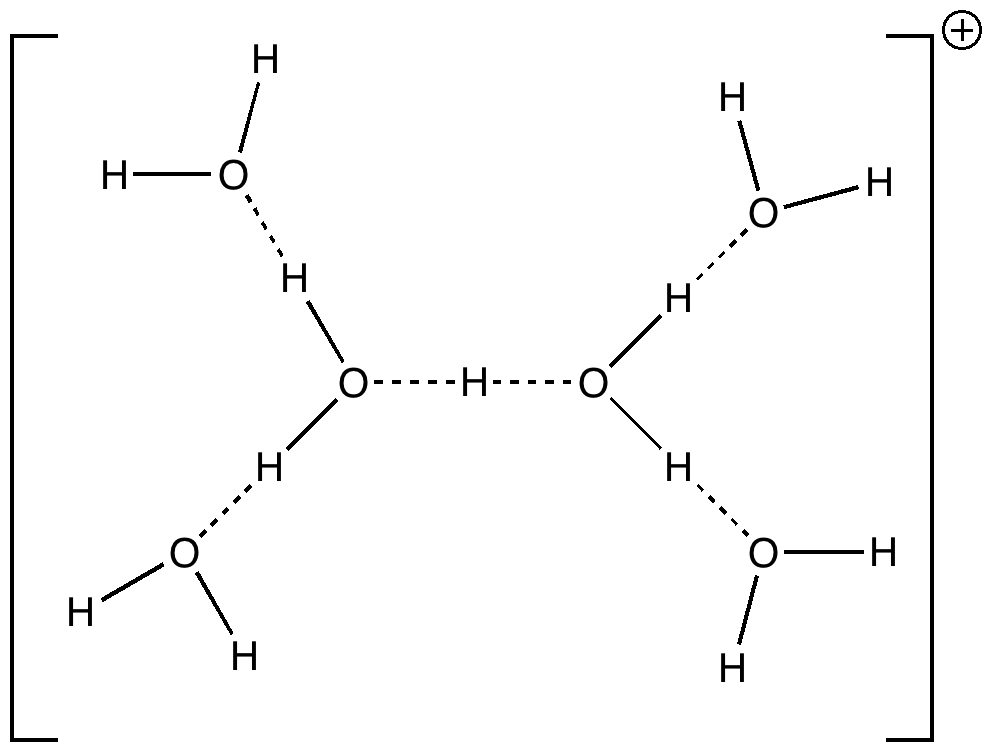
Stoyanov, Stoyanova and Reed recently published on the structure of the hydrogen ion in water.

In an earlier post, I re-visited the conformational analysis of cyclohexane by looking at the vibrations of the entirely planar form (of D 6h symmetry). The method also gave interesting results for the larger cyclo-octane ring. How about a larger leap into the unknown? Let us proceed as follows. One fun game to play in chemistry is to invoke i so-electronic substitutions.
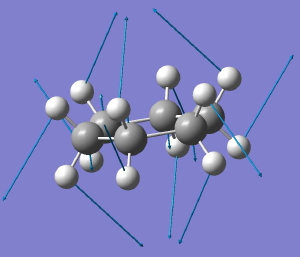
Like benzene, its fully saturated version cyclohexane represents an icon of organic chemistry. By 1890, the structure of planar benzene was pretty much understood, but organic chemistry was still struggling somewhat to fully embrace three rather than two dimensions. A grand-old-man of organic chemistry at the time, Adolf von Baeyer, believed that cyclohexane too was flat, and what he said went.

So ingrained is the habit to think of a bond as a simple straight line connecting two atoms, that we rarely ask ourselves if they are bent, and if so, by how much (and indeed, does it matter?). Well Hursthouse, Malik, and Sales, as long ago as 1978, asked just such a question about the unlikeliest of bonds, a quadruple Cr-Cr bond, found in the compound di-μ-trimethylsilylmethyl-bis-[(tri-methylphosphine)
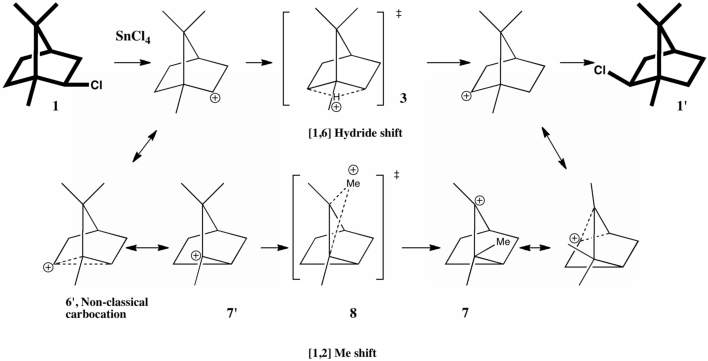
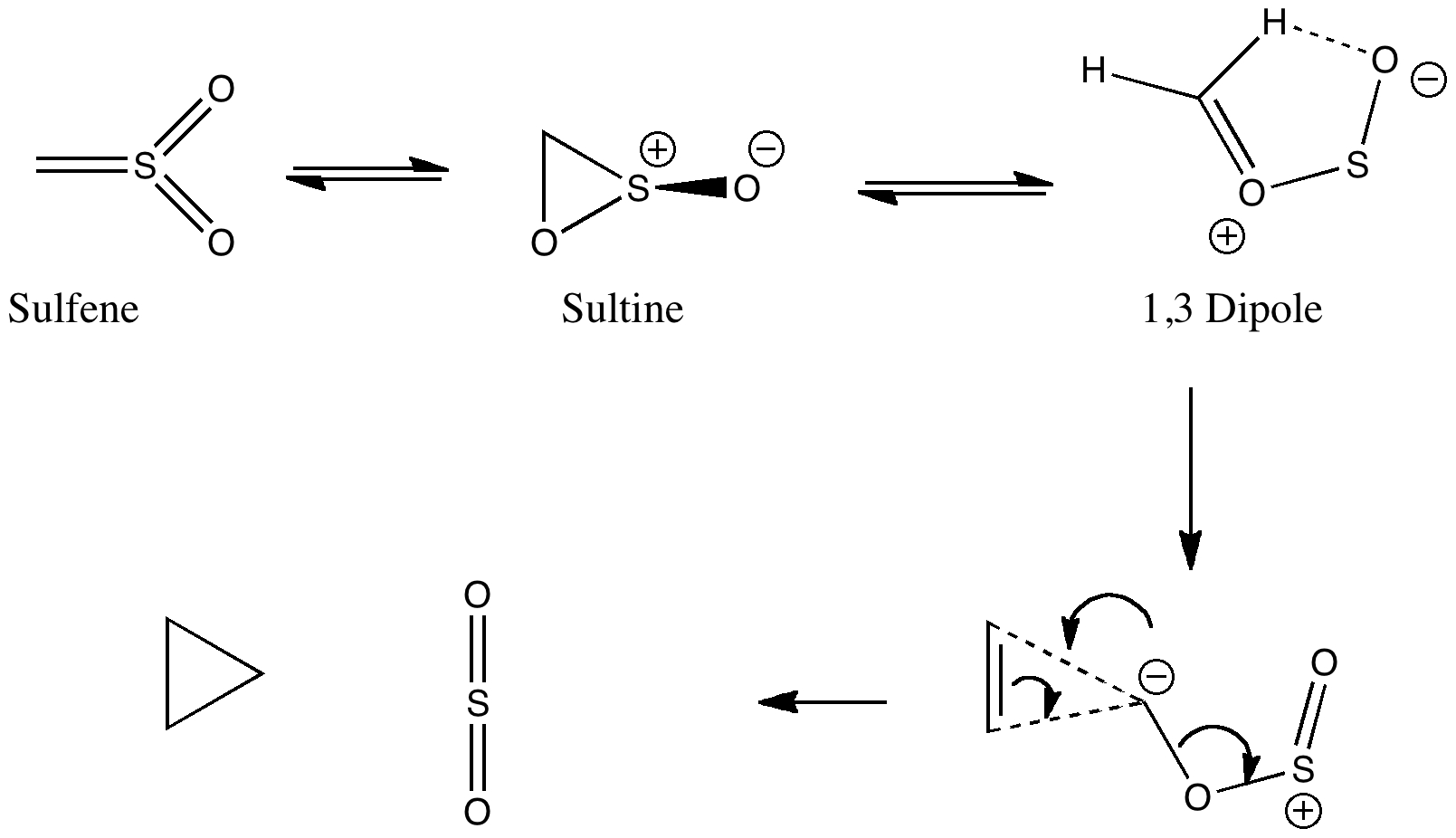
The scheme below illustrates one of the iconic reactions in organic chemistry.

In the previous post, the molecule F3S-C≡SF3 was found to exhibit a valence bond isomerism, one of the S-C bonds being single, the other triple, and with a large barrier (~31 kcal/mol, ν 284i cm-1) to interconversion of the two valence-bond forms.

A previous post posed the question; during the transformation of one molecule to another, what is the maximum number of electron pairs that can simultaneously move either to or from any one atom-pair bond as part of the reaction?