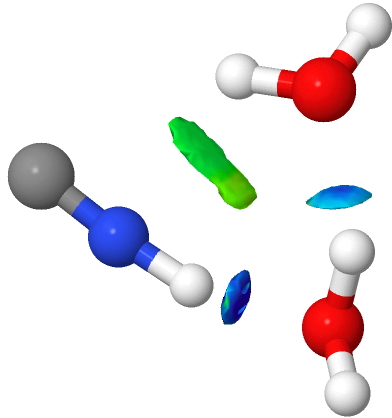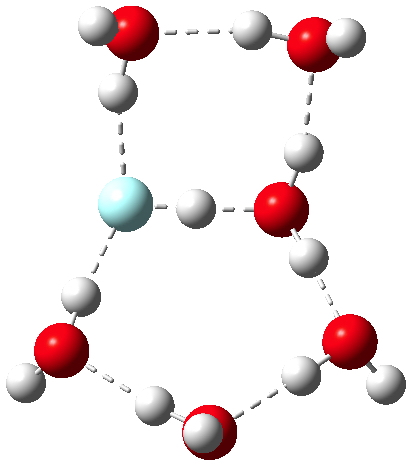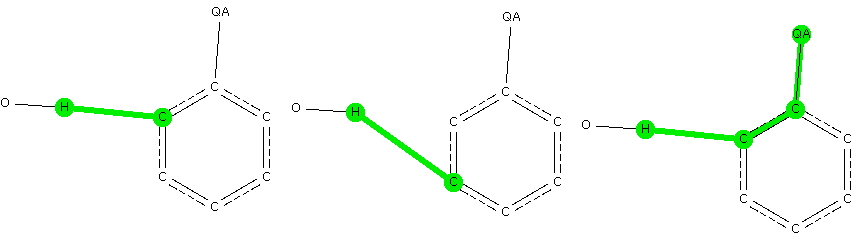
The knowledge that substituents on a benzene ring direct an electrophile engaged in a ring substitution reaction according to whether they withdraw or donate electrons is very old. Introductory organic chemistry tells us that electron donating substituents promote the ortho and para positions over the meta.