The following is a short question in a problem sheet associated with introductory organic chemistry.

The following is a short question in a problem sheet associated with introductory organic chemistry.
The concept of kinetic vs thermodynamic control of a reaction is often taught in the context of the enolisation of e.g. 1-methylcyclohexanone as induced by a base.
The element silicon best represents the digital era of the mid 20th century to the present;
The 1H NMR spectrum of an aromatic molecule such as benzene is iconic; one learns that the unusual chemical shift of the protons (~δ 7-8 ppm) is due to their deshielding by a diatropic ring current resulting from the circulation of six aromatic π-electrons following the Hückel 4n+2 rule.
Homoaromaticity is a special case of aromaticity in which π-conjugation is interrupted by a single sp3 hybridized carbon atom (it is sometimes referred to as a suspended π-bond with no underlying σ-foundation). But consider the carbene shown below. This example comes from a recently published article which was highlighted on Steve Bachrach’s blog.
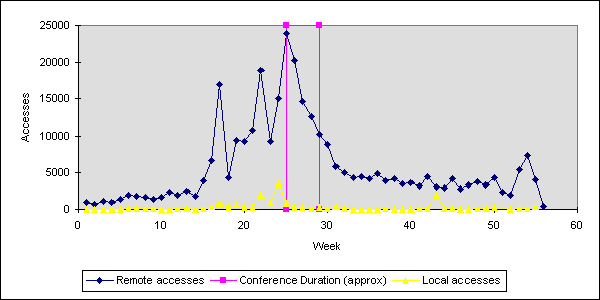
I reminisced about the wonderfully naive but exciting Web-period of 1993-1994. This introduced the server-log analysis to us for the first time, and hits-on-a-web-page.
In 1993-1994, when the Web (synonymous in most minds now with the Internet) was still young, the pace of progress was so rapid that some wag worked out that one “web-year” was like a dog-year, worth about 7 years of normal human time. So in this respect, 1994 is now some 133 web-years ago.
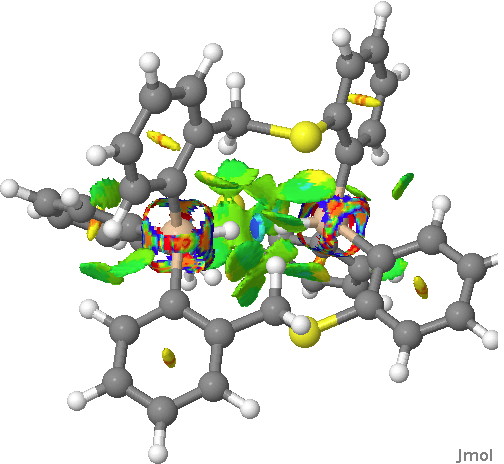
This is a continuation of the discussion started on Steve Bachrach’s blog about a molecule with a very short H…H interaction involving two Si-H groups with enforced proximity. It had been inferred from the X-ray structure that the H…H distance was in the region of 1.50Å. It’s that cis-butene all over again!
In the two-publisher model I proposed a post or so back, I showed an example of how data can be incorporated (transcluded) into the story narrative of a scientific article, with both that story and the data each having their own independently citable reference (using a doi for the citation). Here I take it a step […]
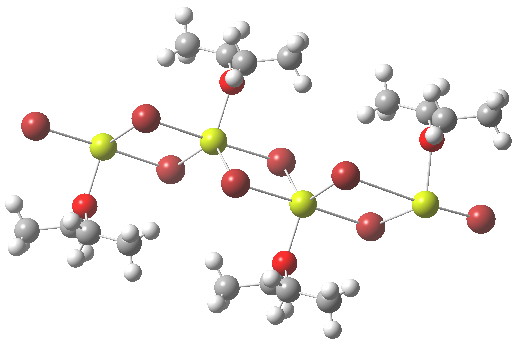
The best known example of the gauche effect is 1,2-difluoroethane, which exhibits a relatively small preference of ~0.5 kcal/mol for this conformer over the anti orientation, which is also a minimum. But FSSF, which I discussed in the previous post, beats this hands down!
Paul Schleyer sent me an email about a pattern he had spotted, between my post on F3SSF and some work he and Michael Mauksch had done 13 years ago with the intriguing title “Demonstration of Chiral Enantiomerization in a Four-Atom Molecule“. Let me explain the connection, but also to follow-up further on what I discovered in […]