This is a follow-up to comment posted by Ryan, who asked about isocyanide’s role (in the form of the anion of tosyl isocyanide, or TosMIC): “In Van Leusen, it (the isocyanide) acts as an electrophile”. The Wikipedia article (recently updated by myself) shows nucleophilic attack by an oxy-anion on the carbon of the C≡N group, […]
The title of this post comes from a comment posted by Ryan, who asks about isocyanide’s role (in the form of the anion of tosyl isocyanide, or TosMIC) in two named reactions, Van Leusen and Ugi FCR.
Here is another example gleaned from that Woodward essay of 1967 (Chem. Soc. Special Publications (Aromaticity), 1967, 21, 217-249), where all might not be what it seems.
Sometimes the originators of seminal theories in chemistry write a personal and anecdotal account of their work. Niels Bohr was one such and four decades later Robert Woodward wrote “The conservation of orbital symmetry” (Chem. Soc. Special Publications (Aromaticity), 1967, 21, 217-249;
In the preceding post, I introduced Dewar’s π-complex theory for alkene-metal compounds, outlining the molecular orbital analysis he presented, in which the filled π-MO of the alkene donates into a Ag+ empty metal orbital and back-donation occurs from a filled metal orbital into the alkene π* MO. Here I play a little “what if” game with this […]
The period 1951–1954 was a golden one for structural chemistry; proteins, DNA, Ferrocene (1952) and the one I discuss here, a bonding model for Zeise’s salt (3).
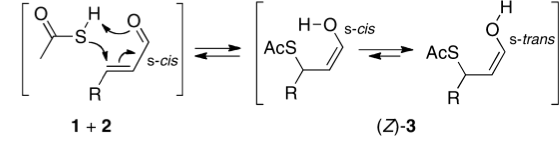
Lukas, who occasionally comments on this blog, sent me the following challenge. In a recent article he had proposed that the stereochemical outcome (Z) of reaction between a butenal and thioacetic acid as shown below arose by an unusual concerted cycloaddtion involving an S-H bond.
The previous post described how the acid catalysed ring opening of propene epoxide by an alcohol (methanol) is preceded by pre-protonation of the epoxide oxygen to form a “hidden intermediate” on the concerted intrinsic reaction pathway to ring opening.
In a previous post on the topic, I remarked how the regiospecific ethanolysis of propene epoxide could be quickly and simply rationalised by inspecting the localized NBO orbital calculated for either the neutral or the protonated epoxide.
A few posts back, I explored the “benzidine rearrangement” of diphenyl hydrazine. This reaction requires diprotonation to proceed readily, but we then discovered that replacing one NH by an O as in N,O-diphenyl hydroxylamine required only monoprotonation to undergo an equivalent facile rearrangement.
I recently got an email from a student asking about the best way of rationalising epoxide ring opening using some form of molecule orbitals. This reminded me of the famous experiment involving propene epoxide.