Michael Dewar famously implicated a so-called π-complex in the benzidine rearrangement, back in the days when quantum mechanical calculations could not yet provide a quantitatively accurate reality check. Because this π-complex actually remains a relatively unusual species to encounter in day-to-day chemistry, I thought I would try to show in a simple way how it forms.
If you search e.g. Scifinder for N,O-diphenyl hydroxylamine (RN 24928-98-1) there is just one literature citation, to a 1962 patent. Nothing else;
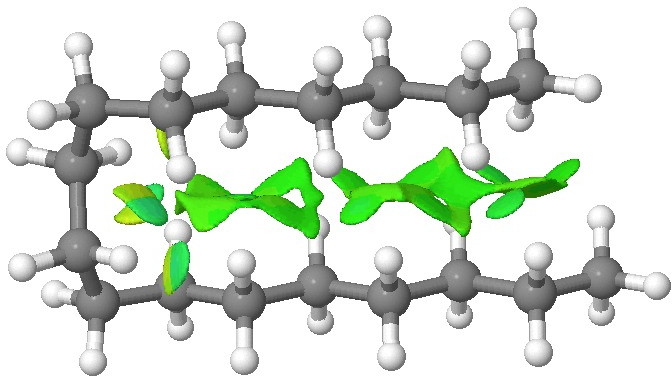
We tend to think of simple hydrocarbons as relatively inert and un-interesting molecules.
Kinetic isotope effects have become something of a lost art when it comes to exploring reaction mechanisms. But in their heyday they were absolutely critical for establishing the mechanism of the benzidine rearrangement. This classic mechanism proceeds via bisprotonation of diphenyl hydrazine, but what happens next was the crux.
This is an interesting result I got when studying the [1,4] sigmatropic rearrangement of heptamethylbicyclo-[3.1.0]hexenyl cations. It fits into the last lecture of a series on pericyclic mechanisms, and just before the first lecture on conformational analysis. This is how they join.
In this post, I looked at some hydrogen bonds formed by interaction of a π-system with an acidic hydrogen. Unlike normal lone pair donors, π-systems can involve more than two electrons, most commonly four or six. Here I look at examples of both these higher-order donors.
Eagle-eyed footnote readers might have spotted one at the bottom of the post on the benzidine rearrangement.
The benzidine rearrangement is claimed to be an example of the quite rare [5,5] sigmatropic migration, which is a ten-electron homologation of the very common [3,3] sigmatropic reaction (e.g. the Cope or Claisen). Some benzidine rearrangements are indeed thought to go through the [3,3] route. The topic has been reviewed here.
A simple correlation between a ring size and the hydrogen bonding as quantified by the O(Lp)/H-O σ* NBO interaction in that ring, indicated a 7- or 8-membered ring was preferred over smaller ones. Here is the same study, but this time using the π-electrons of an alkene as the electron donor.

I previously blogged about anomeric effects involving π electrons as donors, and my post on the conformation of 1,2-difluorethane turned out one of the most popular. Here I thought I would present the results of searching the Cambridge crystal database for examples of the gauche effect.
One frequently has to confront the question: will a hydrogen bond form between a suitable donor (lone pair or π) and an acceptor? One of the factors to be taken into consideration for hydrogen bonds which are part of a cycle is the ring size.