I was intrigued by one aspect of the calculated transition state for di-imide reduction of an alkene; the calculated NMR shieldings indicated an diatropic ring current at the centre of the ring, but very deshielded shifts for the hydrogen atoms being transferred. This indicated, like most thermal pericyclic reactions, an aromatic transition state.
Not a few posts on this blog dissect the mechanisms of well known text-book reactions. But one reaction type where there are few examples on these pages are reductions. These come in three types; using electrons, using a hydride anion and using di-hydrogen.
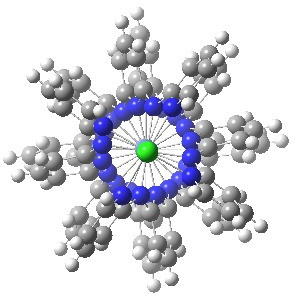
More than 60 million molecules are known, and many are fascinating. But beauty is in the eye of the beholder. Thus it was that I came across the attached molecule. It struck me immediately as, well, beautiful!
A game chemists often play is to guess the mechanism for any given reaction. I thought I would give it a go for the decomposition of the tris-peroxide shown below.
Thalidomide is a chiral molecule, which was sold in the 1960s as a sedative in its (S,R)-racemic form. The tragedy was that the (S)-isomer was tetragenic, and only the (R) enantiomer acts as a sedative.
The text books say that cyclohexenone A will react with a Grignard reagent by delivery of an alkyl (anion) to the carbon of the carbonyl (1,2-addition) but if dimethyl lithium cuprate is used, a conjugate 1,4-addition proceeds, to give the product B shown below.
When methyl manganese pentacarbonyl is treated with carbon monoxide in e.g. di-n-butyl ether, acetyl manganese pentacarbonyl is formed. This classic experiment conducted by Cotton (of quadruple bond fame) and Calderazzo in 1962 dates from an era when chemists conducted extensive kinetic analyses to back up any mechanistic speculations. Their suggested transition state is outlined below.
Following on from our first mechanistic reality check, we now need to verify how product A might arise in the mechanism shown below, starting from B.
The reaction described in the previous post (below) is an unusual example of nucleophilic attack at an sp2-carbon centre, reportedly resulting in inversion of configuration. One can break it down to a sequence of up to eight individual steps, which makes teaching it far easier. But how real is that sequence?
The reaction below plays a special role in my career. As a newly appointed researcher (way back now), I was asked to take tutorial groups for organic chemistry as part of my duties. I sat down to devise a suitable challenge for the group, and came upon the following reaction.
Every once in a while, one encounters a molecule which instantly makes an interesting point. Thus Ruthenium is ten electrons short of completing an 18-electron shell, and it can form a complex with benzene on one face and a ligand known as trimethylenemethane on the other.