Singleton and co-workers have produced some wonderful work showing how dynamic effects and not just transition states can control the outcome of reactions. Steve Bachrach’s blog contains many examples, including this recent one.
Years ago, I was travelling from Cambridge to London on a train. I found myself sitting next to a chemist, and (as chemists do), he scribbled the following on a piece of paper. When I got to work the next day Vera (my student) was unleashed on the problem, and our thoughts were published.
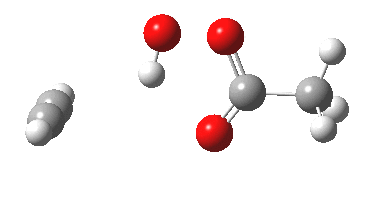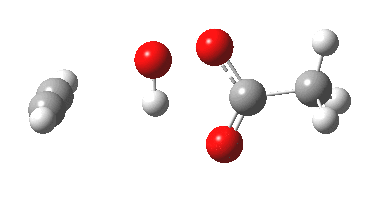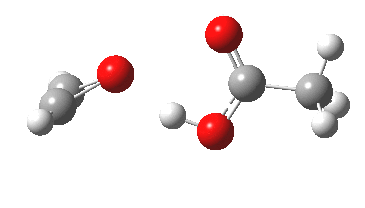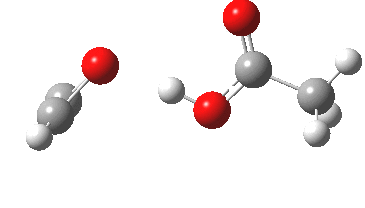
Sometimes, connections between different areas of chemistry just pop out (without the help of semantic web tools, this is called serendipity). So here, I will try to join up some threads which emerge from previous posts.
Back in 1994, we published the crystal structure of the molecule below (X=H), a putative anti-malarial drug called halofantrine. Little did we realise that a whole area of organo catalysis based on a thiourea catalyst with a similar motif would emerge a little later. Here is how the two are connected.
The reaction between a carbene and an alkene to form a cyclopropane is about as simple a reaction as one can get. But I discussed before how simple little molecules (cyclopropenyl anion) can hold surprises.
This is a continuation of the previous post exploring the transition state geometries of various types of ring closure as predicted by Baldwin’s rules. I had dealt with bond formation to a trigonal (sp2) carbon; now I add a digonal (sp) example (see an interesting literature variation).
The Baldwin rules for ring closure follow the earlier ones by Bürgi and Dunitz in stating the preferred angles of nucleophilic (and electrophilic) attack in bond forming reactions, and are as famous for the interest in their exceptions as for their adherence.
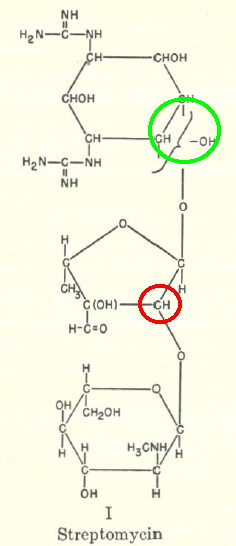
Streptomycin is an antibiotic active against tuberculosis, and its discovery has become something of a cause célèbre. It was first isolated on October 19, 1943 by a graduate student Albert Schatz in the laboratory of Selman Waksman at Rutgers University. I want to concentrate in this post on its molecular structure.
If you have not previously visited, take a look at Nick Greeves’ ChemTube3D , an ever-expanding gallery of reactions and their mechanisms. The 3D is because all molecules are offered with X, Y and z coordinates. You also get arrow pushing‡ in 3D. Here, I argue that we should adopt Einstein, and go to the space-time continuum!
HCl reacting with a carbonyl compound (say formaldehyde) sounds pretty simple. But often the simpler a thing looks, the more subtle it is under the skin. And this little reaction is actually my prelude to the next post.
Many reaction mechanisms involve a combination of bond formation/cleavage between two non-hydrogen atoms and those involving reorganisation of proximate hydrogens. The Baeyer-Villiger discussed previously illustrated a complex dance between the two types. Here I take a look at another such mechanism, the methylation of a carboxylic acid by diazomethane.