How one might go about answering the question: do alkenes promote anomeric effects?
My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down.
Most of the chemical structure diagrams in this blog originate from Chemdraw, which seems to have been around since the dawn of personal computers!
Moore’s law describes a long-term trend in the evolution of computing hardware, and it is often interpreted in terms of processing speed. Here I chart this rise in terms of the size of computable molecules.
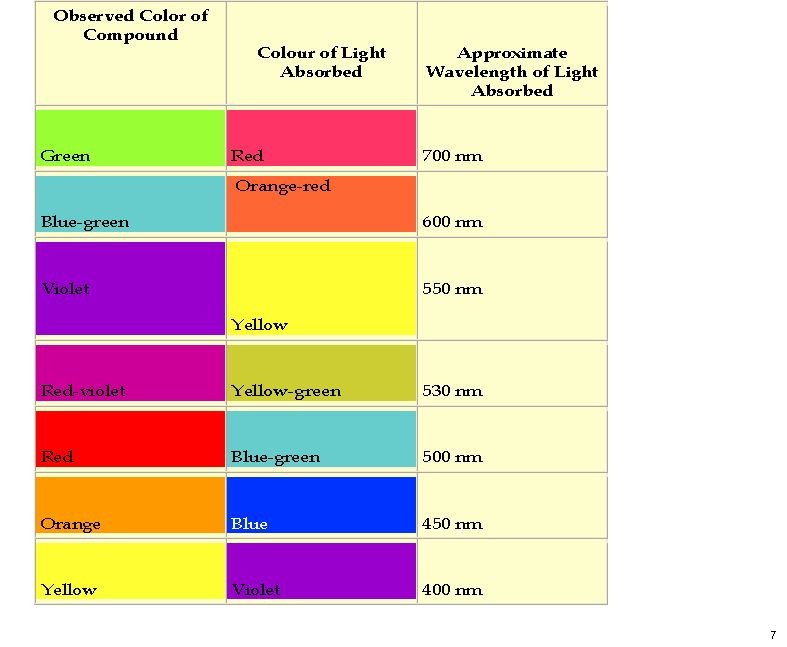
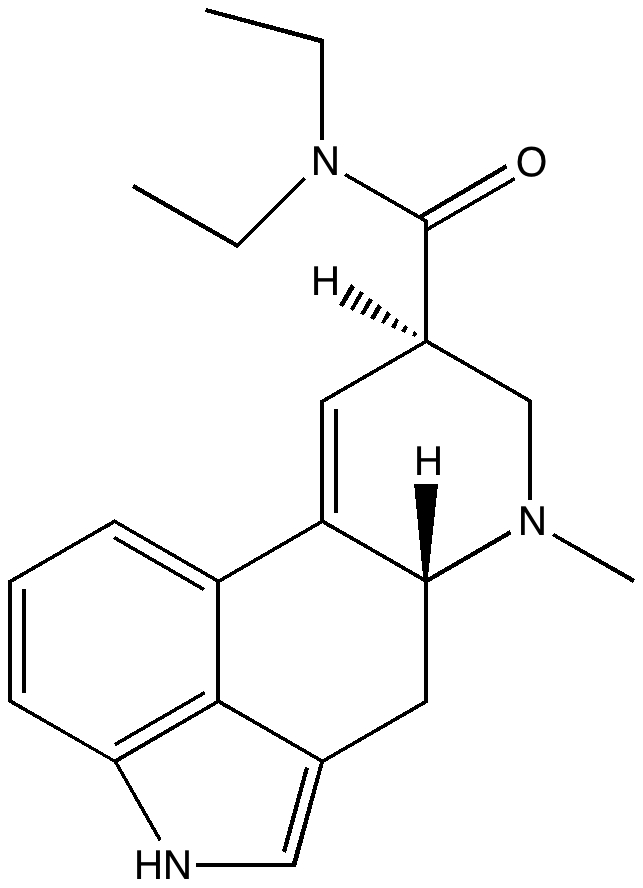
My previous post on the topic of mauveine left the outcome dangling. Put simply, λmax is measured at about 549nm for mauveine A, but was calculated at about 440nm using a modern method for predicting colour (TD-DFT). According to the colour table below, that would make it orange, not mauve.
As the title hints, I have been here before. The SN1 solvolysis mechanism of t-butyl chloride was central to the flourishing of physical organic chemistry from the 1920s onwards, and it appears early on in most introductory lecture courses and text books. There we teach that it is a two-stage mechanism.
I have for perhaps the last 25 years been urging publishers to recognise how science publishing could and should change.
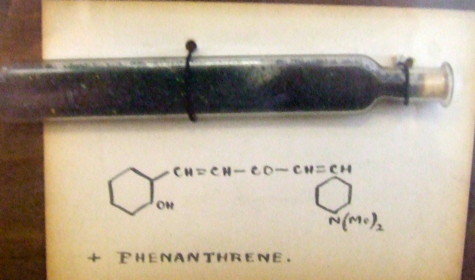
Organic chemists have been making (more or less pure) molecules for the best part of 180 years. Occasionally, these ancient samples are unearthed in cupboards, and then the hunt for their origin starts. I have previously described tracking down the structure of a 120 year-old sample of a naphthalene derivative.
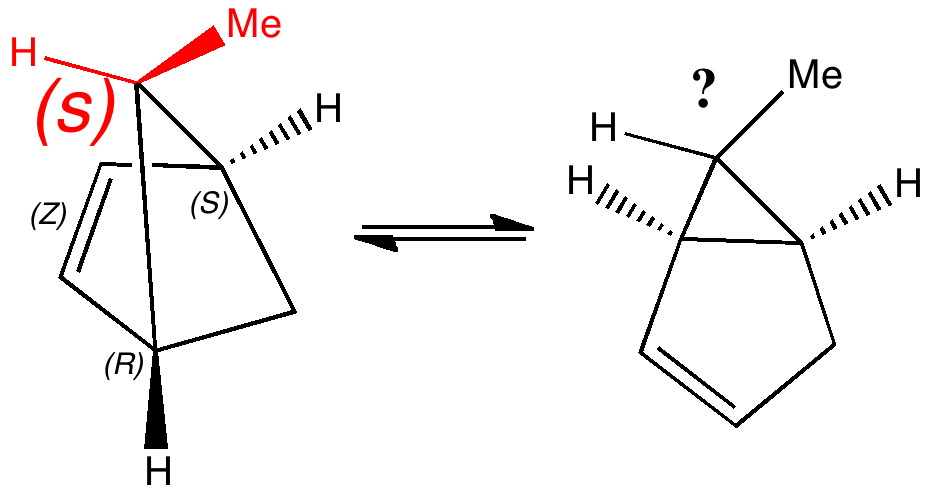
Most representational chemistry generated on a computer requires the viewer to achieve a remarkably subtle transformation in their mind from two to three dimensions (we are not quite yet in the era of the 3D iPad!). The Cahn-Ingold-Prelog convention was a masterwork (which won the Nobel prize). It is shown in action for the molecule […]
I asked a while back whether blogs could be considered a serious form of scholarly scientific communication (and so has Peter Murray-Rust more recently). A case for doing so might be my post of about a year ago, addressing why borane reduces a carboxylic acid, but not its ester, where I suggested a possible mechanism.
Bonds are a good example of something all chemists think they can recognise when they see them. But they are also remarkably dependent on context.