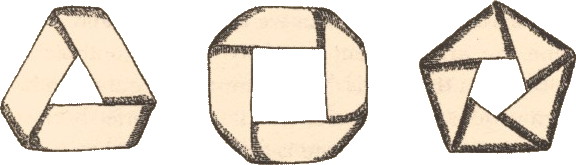
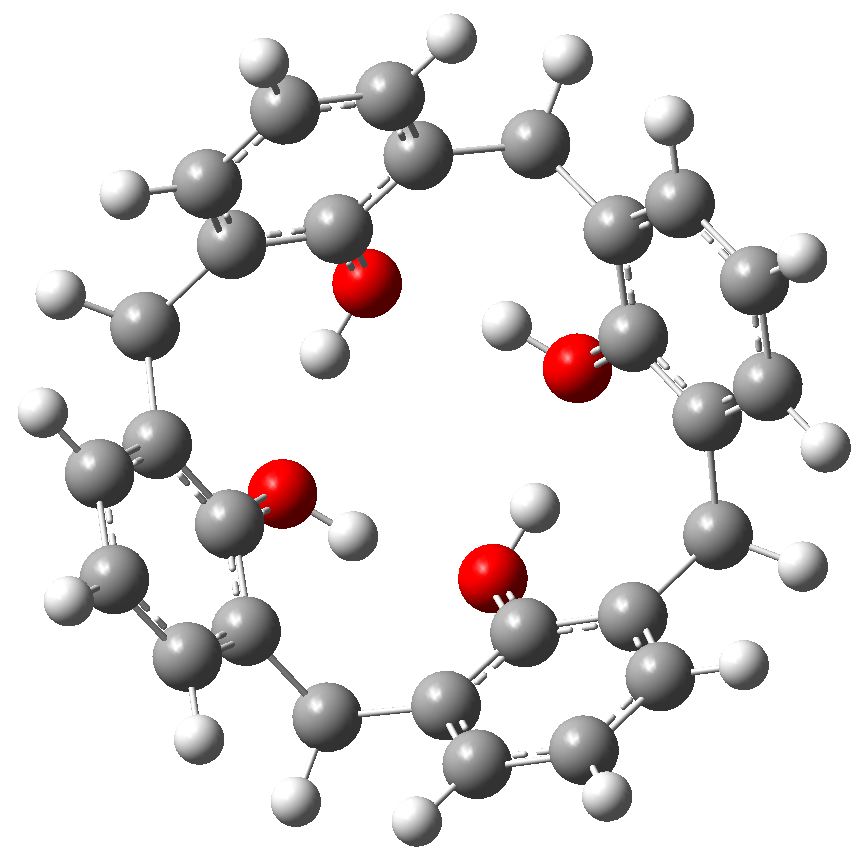
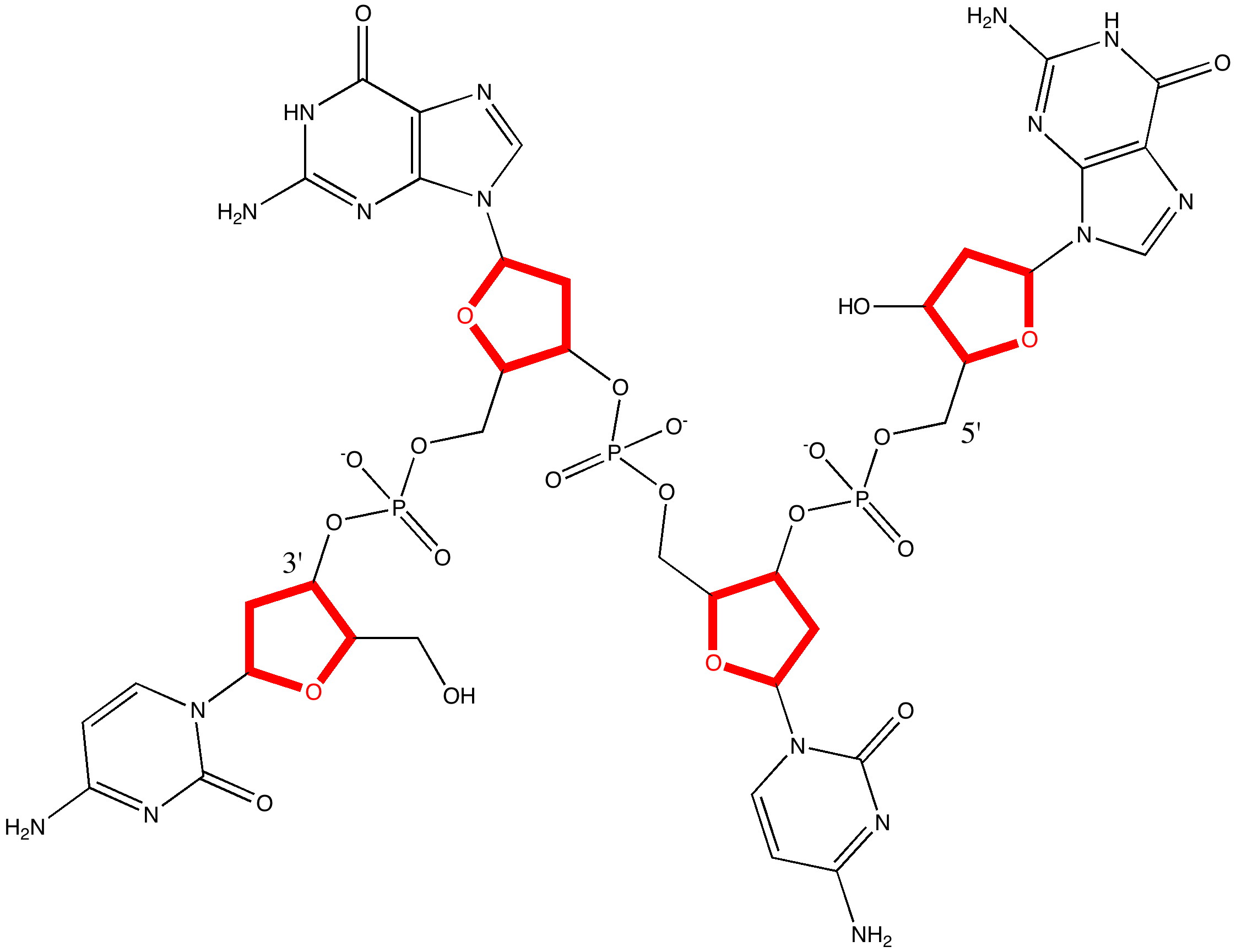
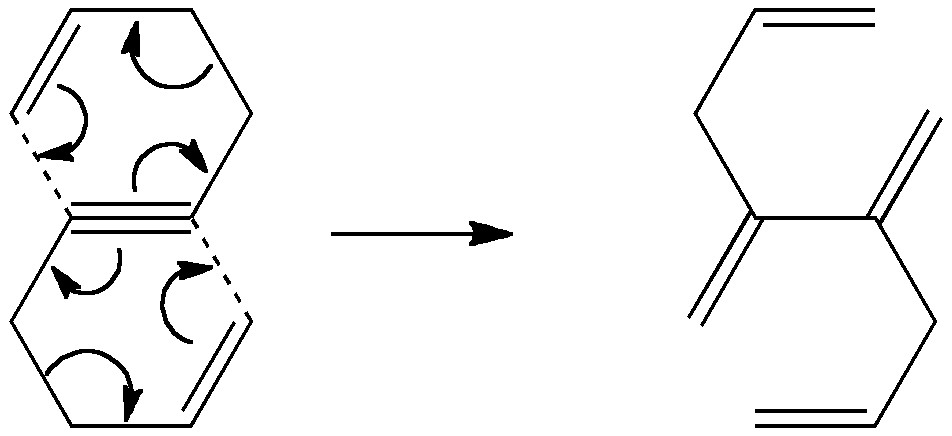
One of my chemical heroes is William Perkin, who in 1856 famously (and accidentally) made the dye mauveine as an 18 year old whilst a student of August von Hofmann, the founder of the Royal College of Chemistry (at what is now Imperial College London). Perkin went on to found the British synthetic dyestuffs and perfumeries industries.