If you get a small rotatable molecule below, then ChemDoodle/HTML5/WebGL is working. Why might this be important? Well, the future is mobile, in other words, devices that rely on batteries or other sources of built-in power. This means the power guzzling GPU cards of the past (some reach ~400 Watts!) cannot be used.
If you visit this blog you will see a scientific discourse in action.

Chemistry gets complex very rapidly. Consider the formula CH3NO as the topic for a tutorial in introductory chemistry. I challenge my group (of about 8 students) to draw as many different molecules as they can using exactly those atoms. I imply that perhaps each of them might find a different structure;
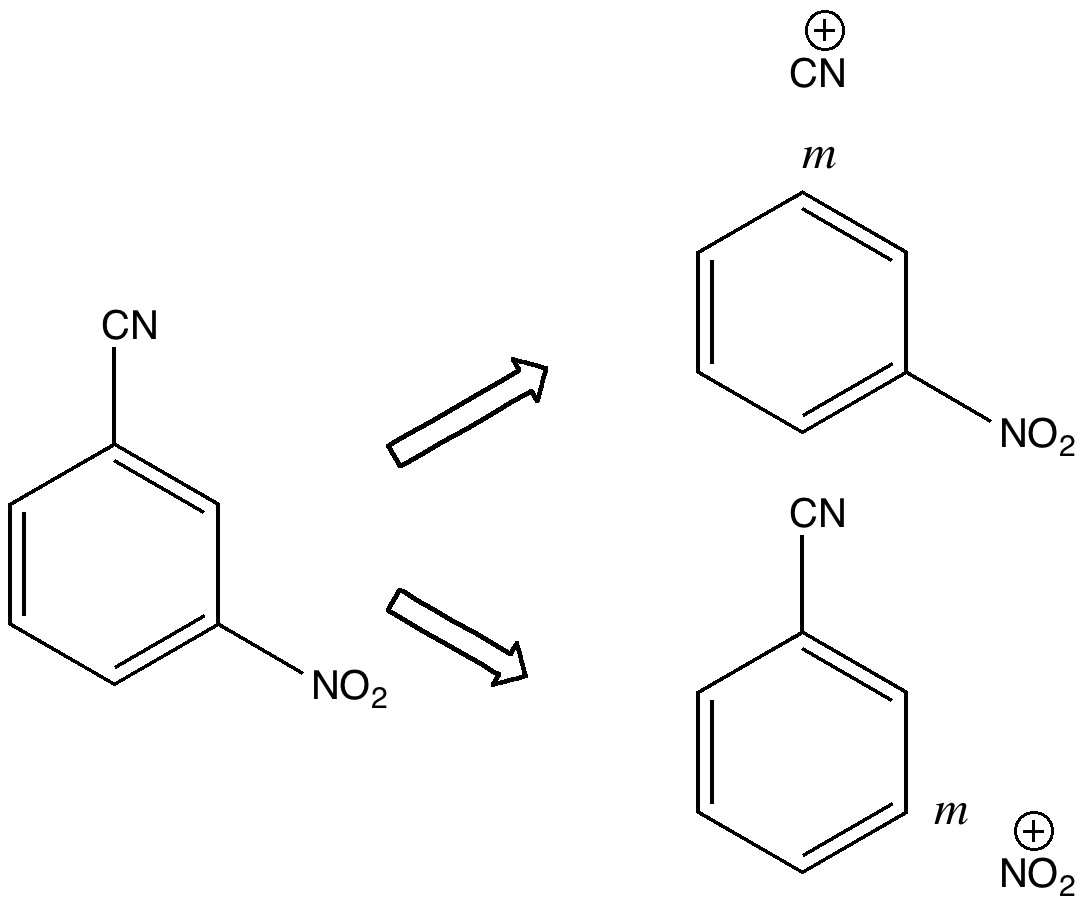
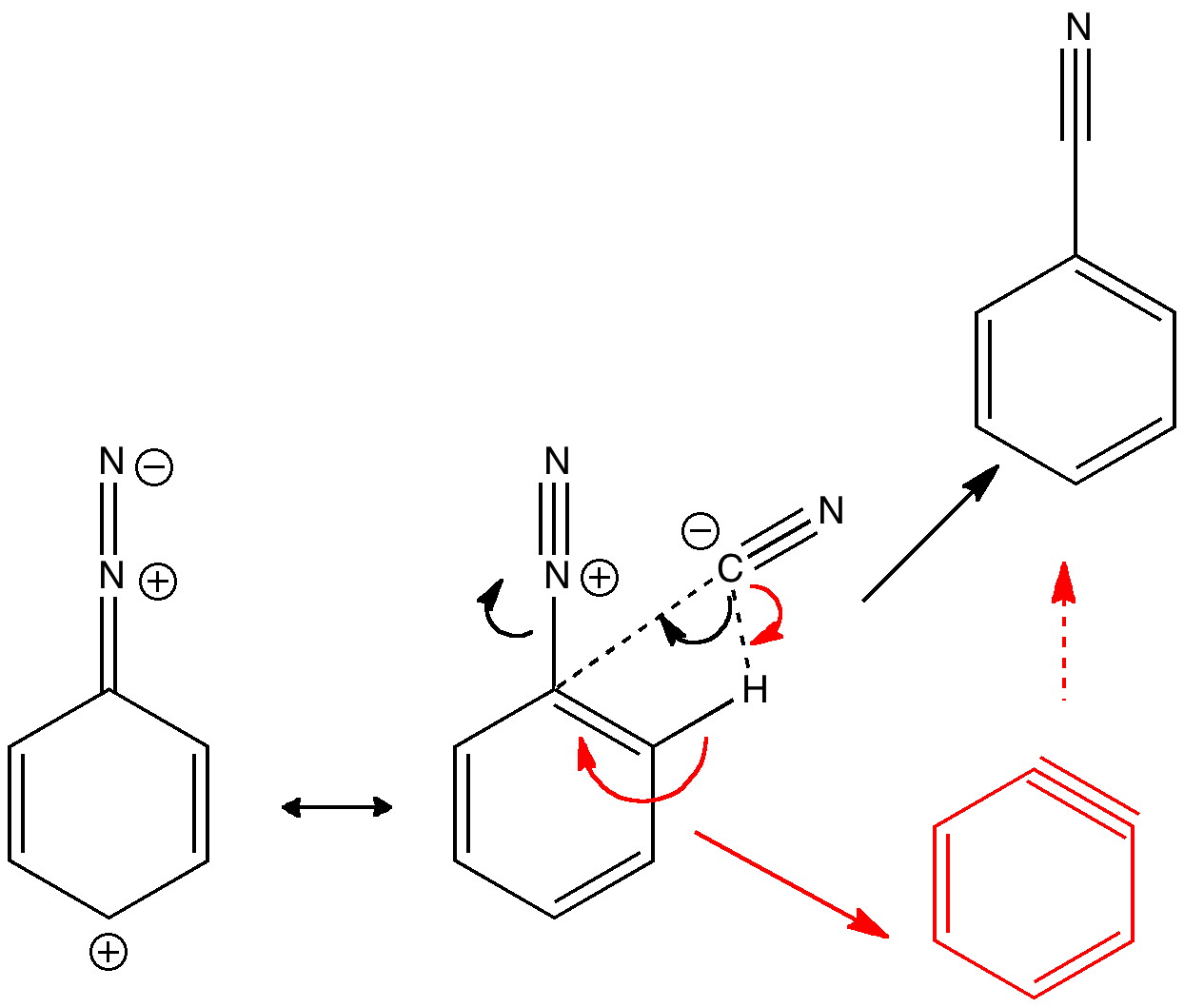
Do you fancy a story going from simplicity to complexity, if not absurdity, in three easy steps? Read on! The following problem appears in one of our (past) examination questions in introductory organic chemistry. From relatively mundane beginnings, one can rapidly find oneself in very unexpected territory.
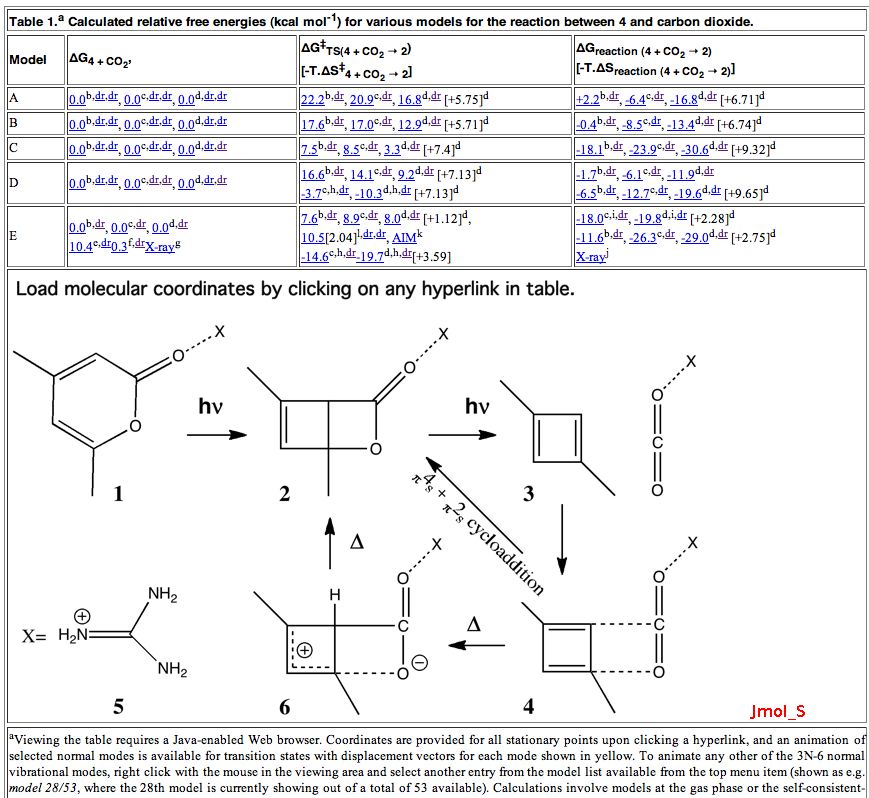
Janus was the mythological Roman god depicted as having two heads facing opposite directions, looking simultaneously into the past and the future. Some of the most ancient (i.e. 19th century) known reactions can be considered part of a chemical mythology; perhaps it is time for a Janus-like look into their future.
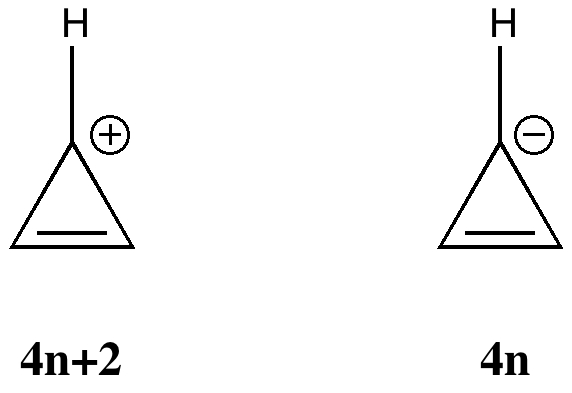
More inspiration from tutorials. In a lecture on organic aromaticity, the 4n+2/4n Hückel rule was introduced (in fact, neither rule appears to have actually been coined in this form by Hückel himself!). The simplest examples are respectively the cyclopropenyl cation and anion.
Moving (chemical) data around in a manner which allows its (automated) use in whichever context it finds itself must be a holy grail for all scientists and chemists.

My university tutorial yesterday covered selective reductions of functional groups in organic chemistry. My thoughts on that topic have now morphed into something rather different. Scientific research has a habit of having this sort of thing happen.
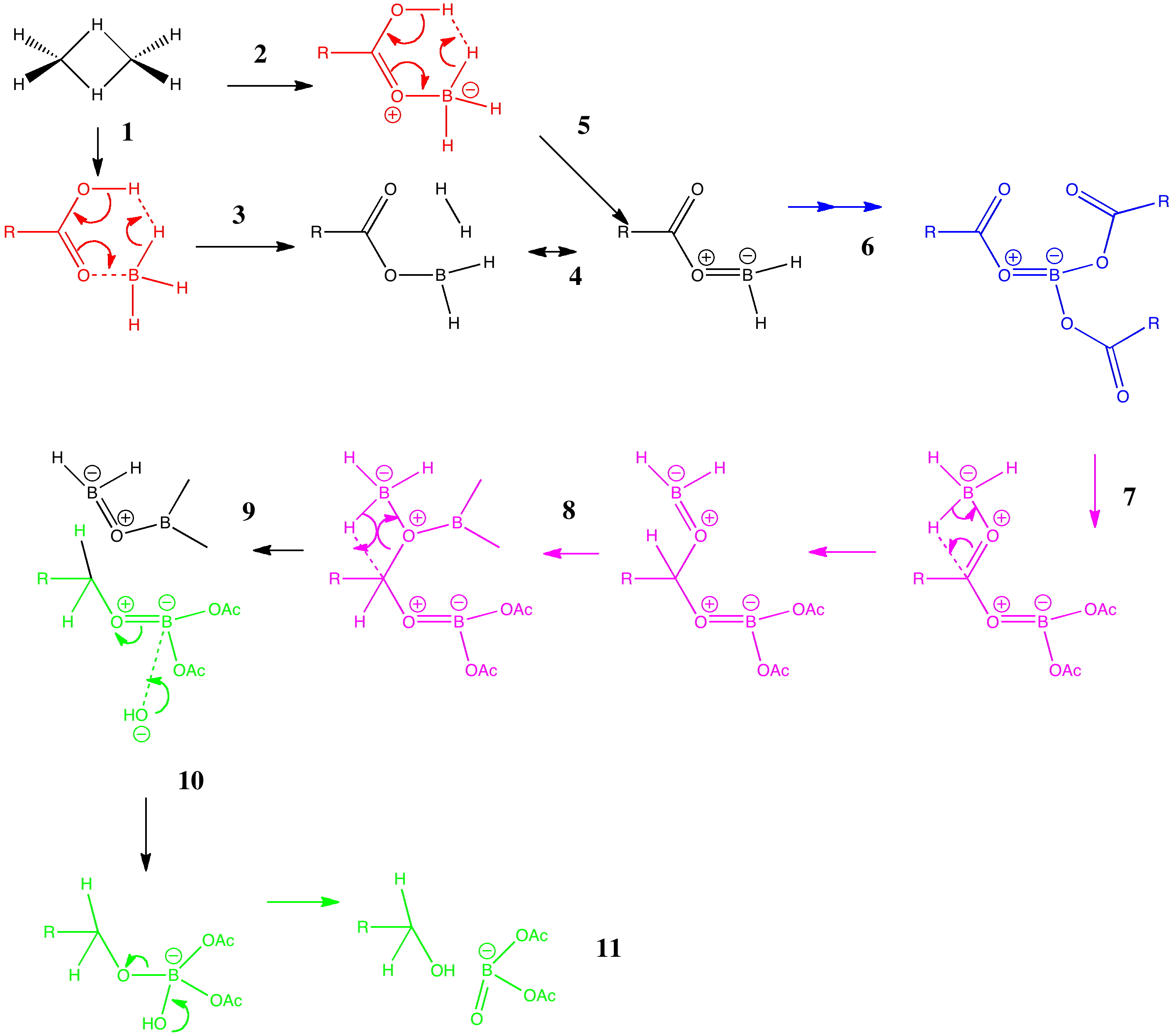
Arrow pushing (why never pulling?) is a technique learnt by all students of organic chemistry (inorganic chemistry seems exempt!). The rules are easily learnt (supposedly) and it can be used across a broad spectrum of mechanism.
For those of us who were around in 1985, an important chemical IT innovation occurred.
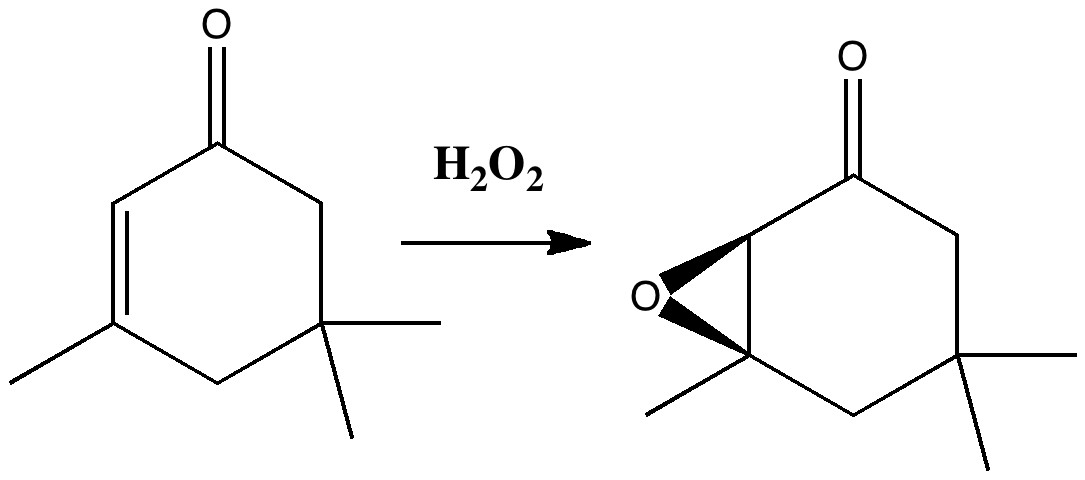
Our understanding of science mostly advances in small incremental and nuanced steps (which can nevertheless be controversial) but sometimes the steps can be much larger jumps into the unknown, and hence potentially more controversial as well.