
The molecule below was characterised in 1996 (DOI: 10.1246/cl.1996.489) and given the name tris(dithiolene)vanadium (IV). No attempt was made in the original article to give this molecule a Lewis structure using Lewis electron pair bonds.

The molecule below was characterised in 1996 (DOI: 10.1246/cl.1996.489) and given the name tris(dithiolene)vanadium (IV). No attempt was made in the original article to give this molecule a Lewis structure using Lewis electron pair bonds.
Many university chemistry departments, and mine is no exception, like to invite applicants to our courses to show them around. Part of the activities on the day is an “interview” in which the candidate is given a chance to shine.
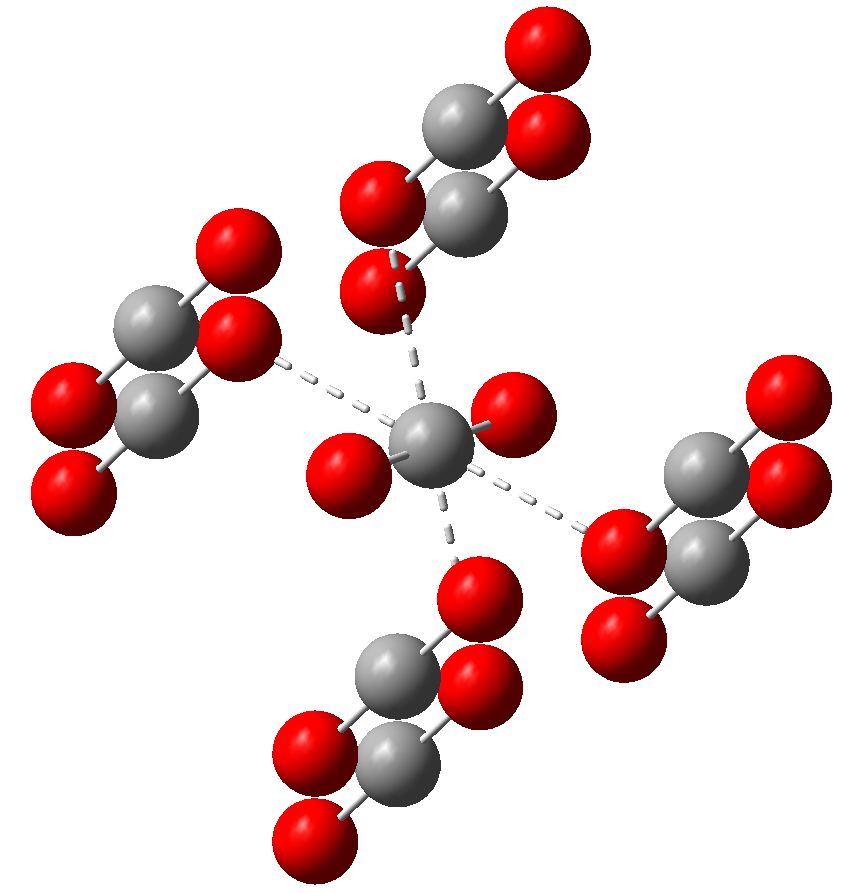
Carbon dioxide is much in the news, not least because its atmospheric concentration is on the increase. How to sequester it and save the planet is a hot topic. Here I ponder its solid state structure, as a hint to its possible reactivity, and hence perhaps for clues as to how it might be captured.
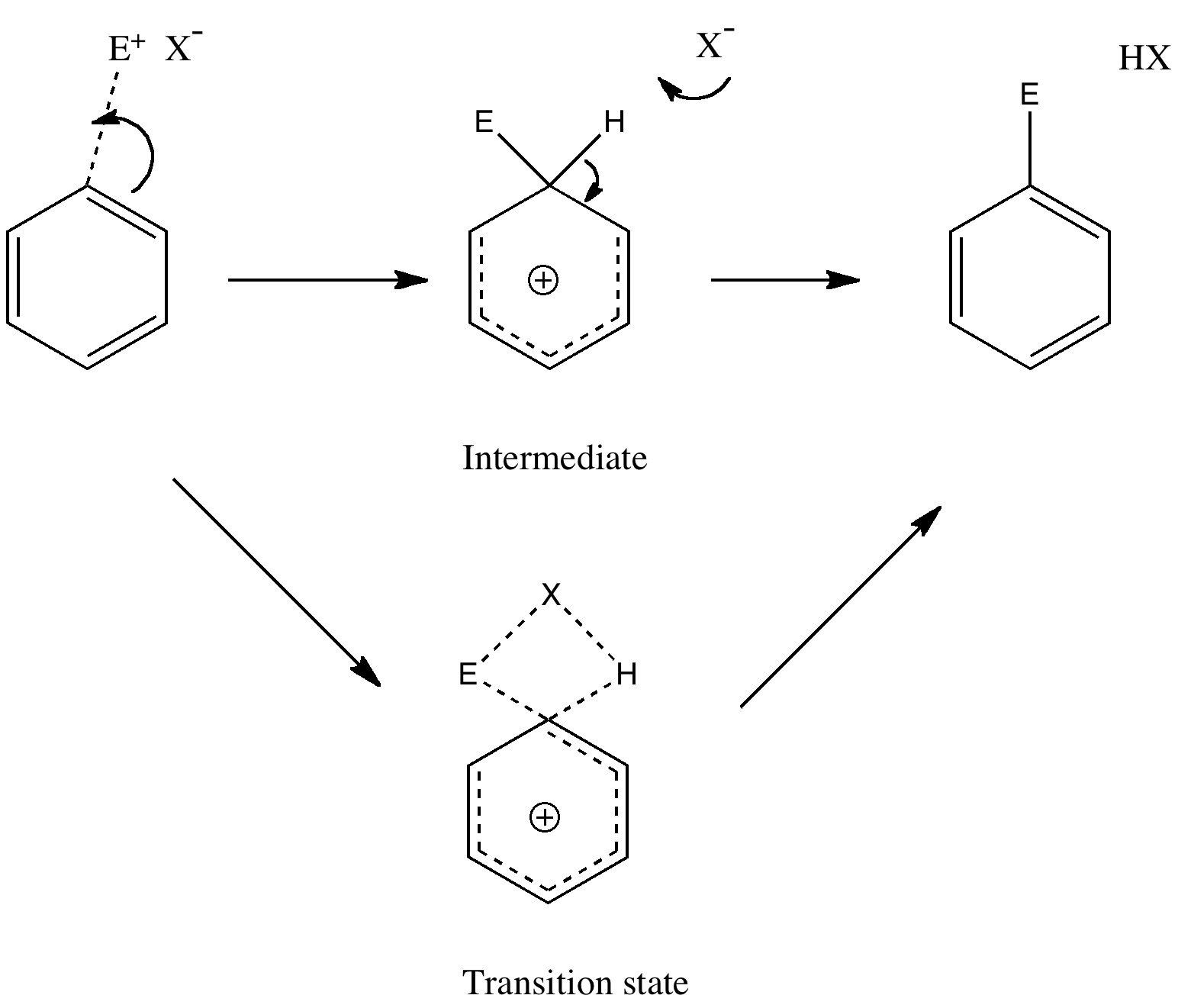
Unravelling reaction mechanisms is thought to be a 20th century phenomenon, coincident more or less with the development of electronic theories of chemistry. Hence electronic arrow pushing as a term. But here I argue that the true origin of this immensely powerful technique in chemistry goes back to the 19th century.

Cavities promote reactions, and they can also trap the products of reactions. Such (supramolecular) chemistry is used to provide models for how enzymes work, but it also allows un-natural reactions to be undertaken.
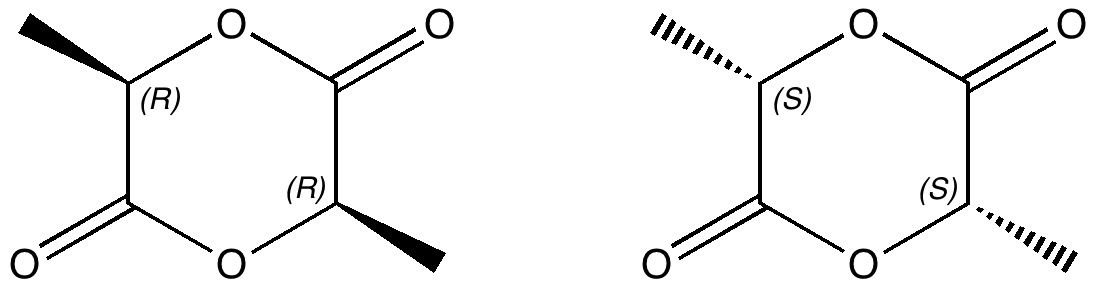
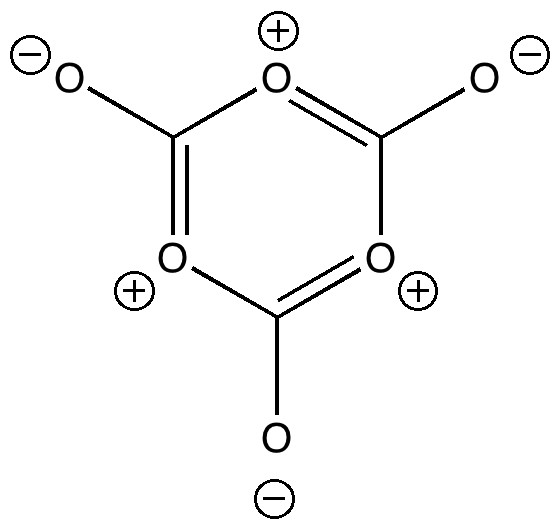
Lactide is a small molecule made from lactic acid, which is itself available in large quantities by harvesting plants rather than drilling for oil. Lactide can be turned into polymers with remarkable properties, which in turn degrade down easily back to lactic acid. A perfect bio-renewable material!
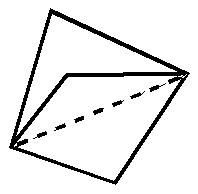
In this post, I will take a look at what must be the most extraordinary small molecule ever made (especially given that it is merely a hydrocarbon). Its peculiarity is the region indicated by the dashed line below. Is it a bond?

NCI (non-covalent interactions) is the name of a fascinating new technique for identifying exactly these. Published recently by Johnson, Keinan, Mori-Snchez, Contreras-Garca, Cohen and Yang, it came to my attention at a conference to celebrate the 20th birthday of ELF when Julia Contreras-Garcia talked about the procedure.

The title of this post merges those of the two previous ones. The tunable C-Cl bond brought about in the molecule tris(amino)chloromethane by anomeric effects will be probed using the Laplacian of the electronic density.

The Cheshire cat in Alice’s Adventures in Wonderland comes and goes at will, and engages Alice with baffling philosophical points. Chemical bonds are a bit like that too.
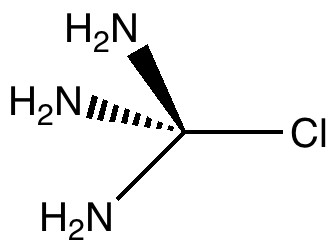
Car transmissions come in two types, ones with fixed ratio gears, and ones which are continuously variable. When it comes to chemical bonds, we tend to think of them as being very much of the first type. Bonds come in fixed ratios; single, aromatic, double, triple, etc.