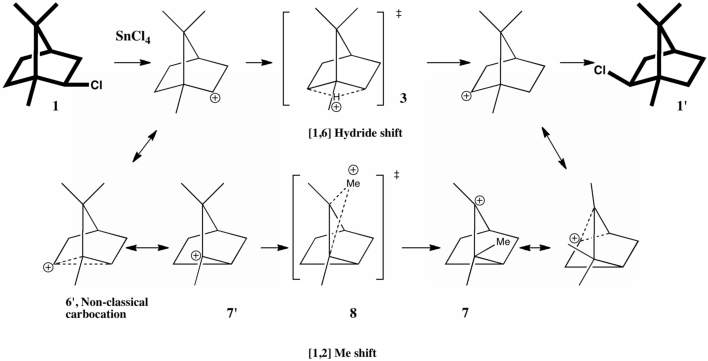
So ingrained is the habit to think of a bond as a simple straight line connecting two atoms, that we rarely ask ourselves if they are bent, and if so, by how much (and indeed, does it matter?). Well Hursthouse, Malik, and Sales, as long ago as 1978, asked just such a question about the […]