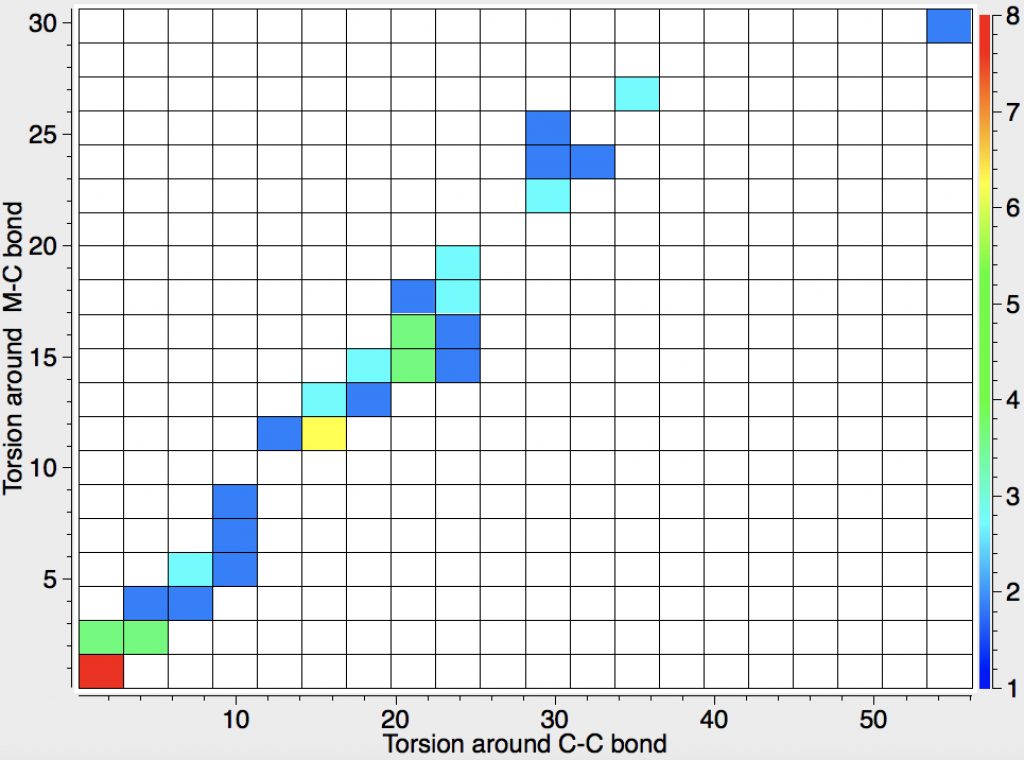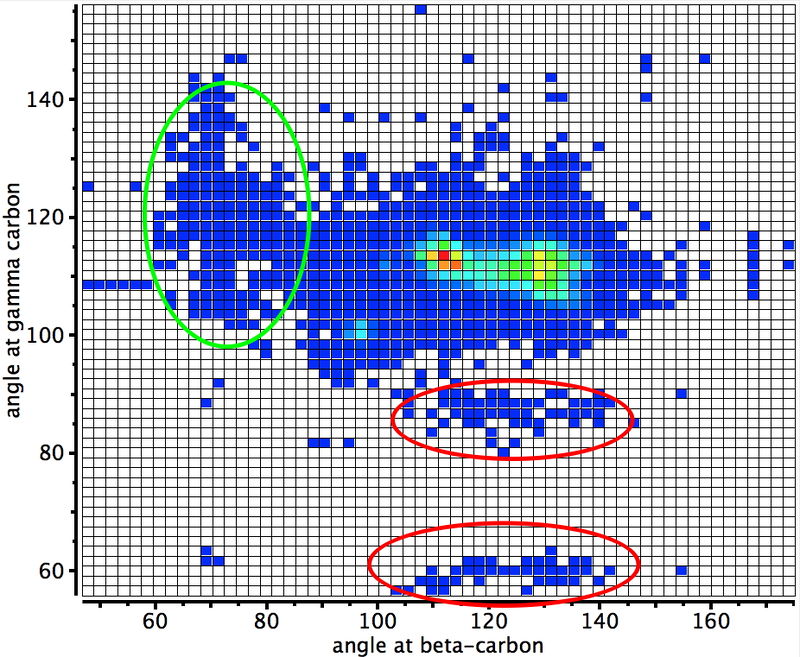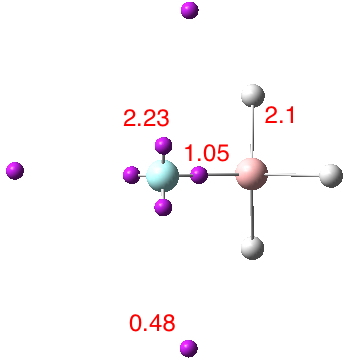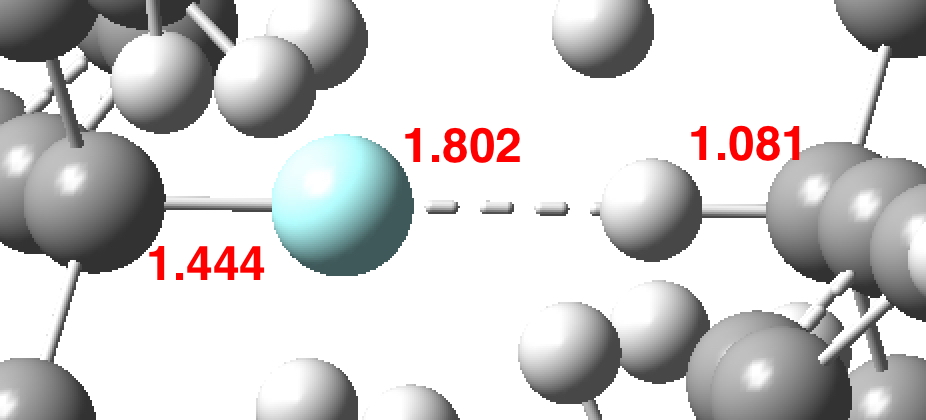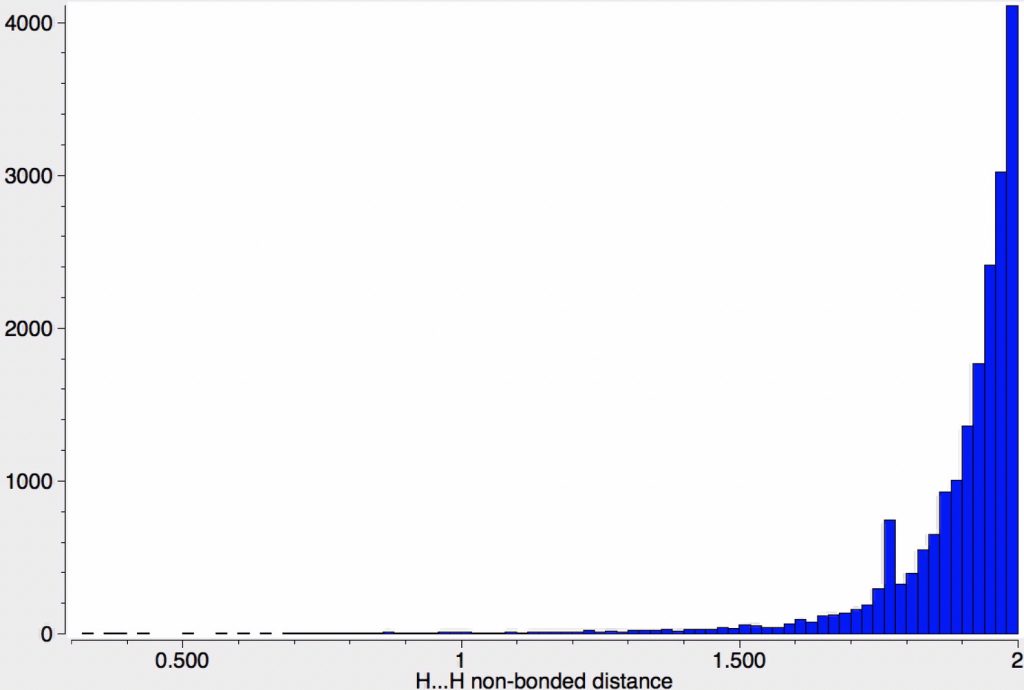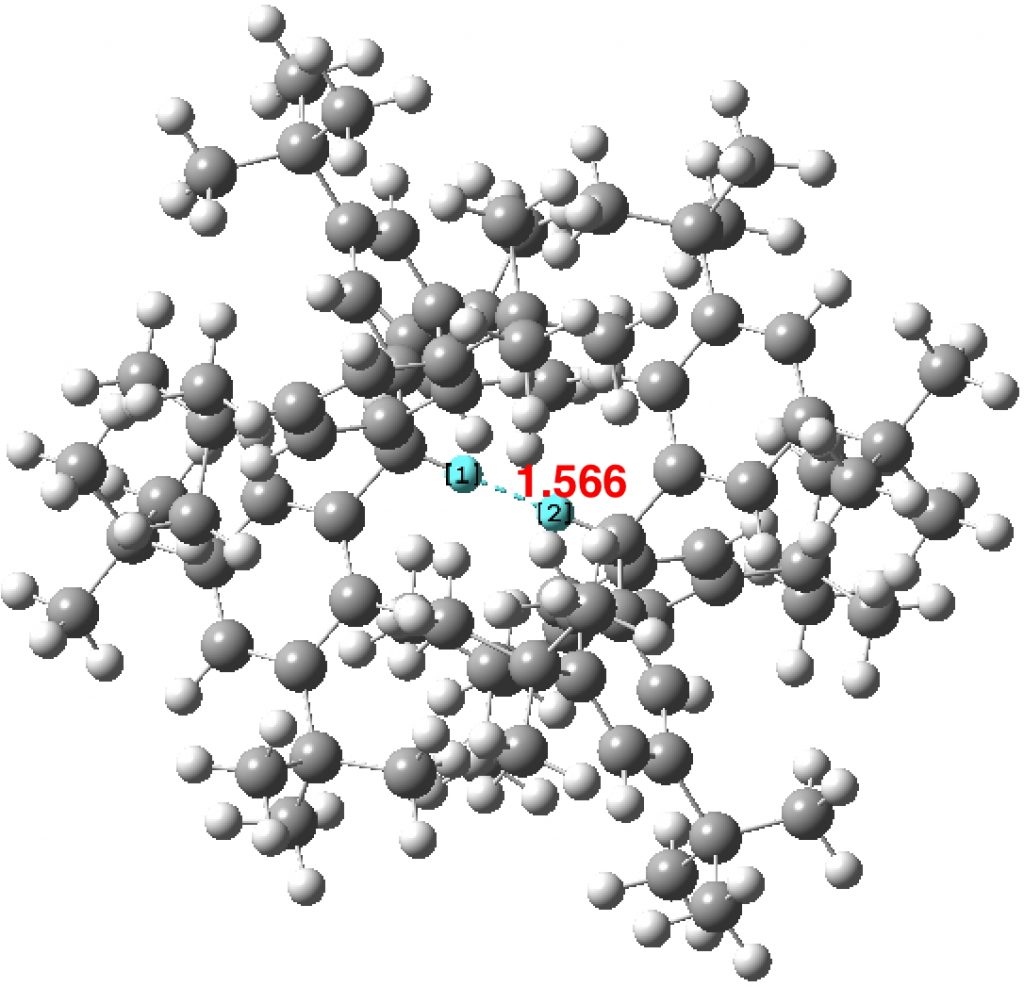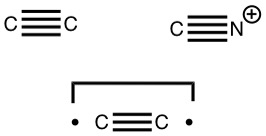
The chemical bond zoo is relatively small (the bond being a somewhat fuzzy concept, I am not sure there is an actual count of occupants). So when two new candidates come along, it is worth taking notice. I have previously noted the Chemical Bonds at the 21st Century-2017: CB2017 Aachen conference, where both were discussed.