On February 6th I was alerted to this intriguing article by a phone call, made 55 minutes before the article embargo was due to be released. Gizmodo wanted to know if I could provide an (almost)† instant‡ quote.

On February 6th I was alerted to this intriguing article by a phone call, made 55 minutes before the article embargo was due to be released. Gizmodo wanted to know if I could provide an (almost)† instant‡ quote.
The book of the title has recently appeared giving a rich and detailed view over 417 pages, four appendices and 24 pages of photographs of how a university chemistry department in the UK came into being in 1845 and its subsequent history of discoveries, Nobel prizes and much more.
The title refers to an upcoming symposium on the topic on 22-24 May, 2017.

Almost exactly 20 years ago, I started what can be regarded as the precursor to this blog. As part of a celebration of this anniversary, I revisited the page to see whether any of it had withstood the test of time. Here I recount what I discovered.
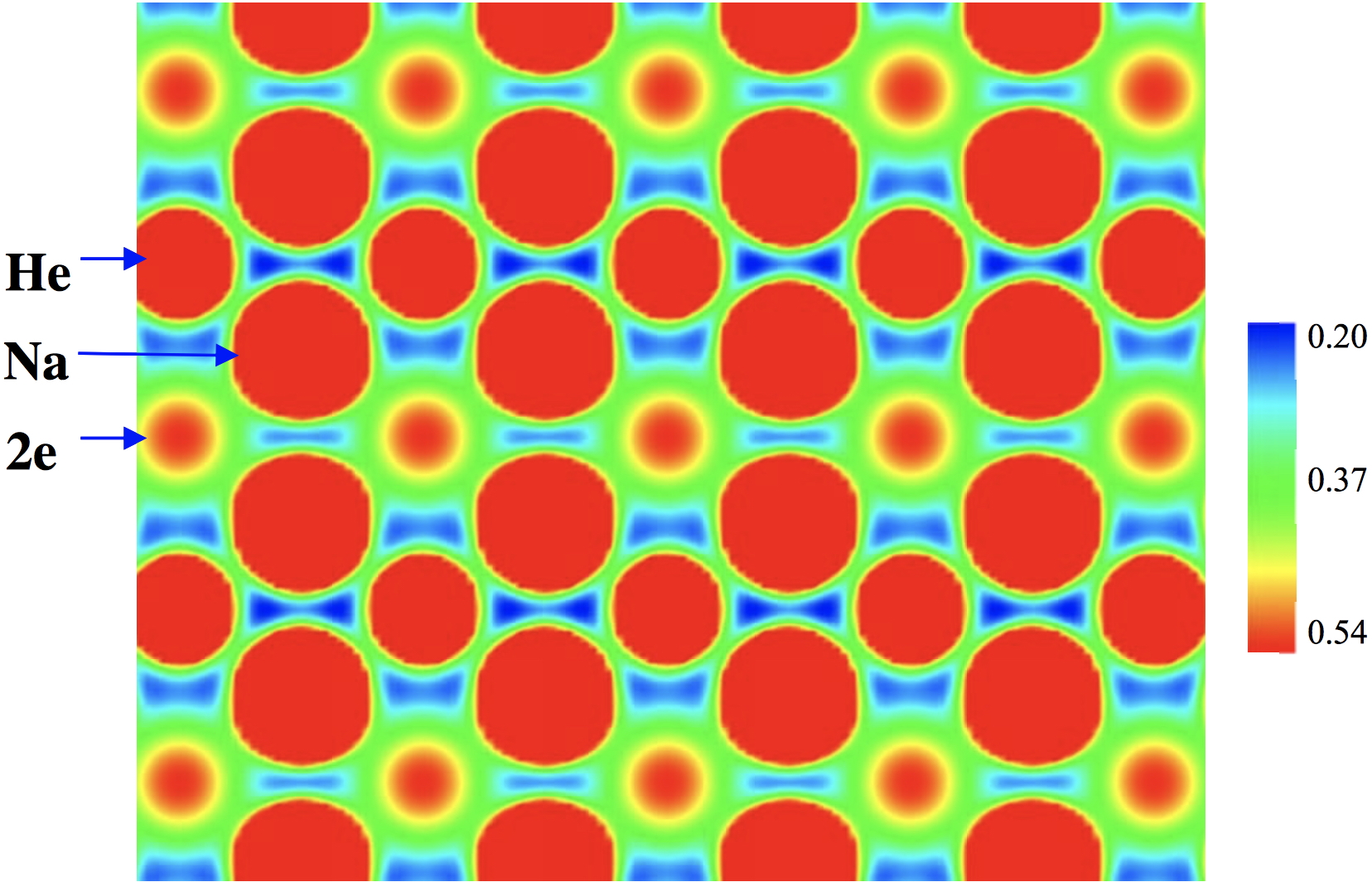
The story so far. Inspired by the report of the most polar neutral compound yet made, I suggested some candidates based on the azulene ring system that if made might be even more polar. This then led to considering a smaller π-analogue of azulene, m-benzyne.
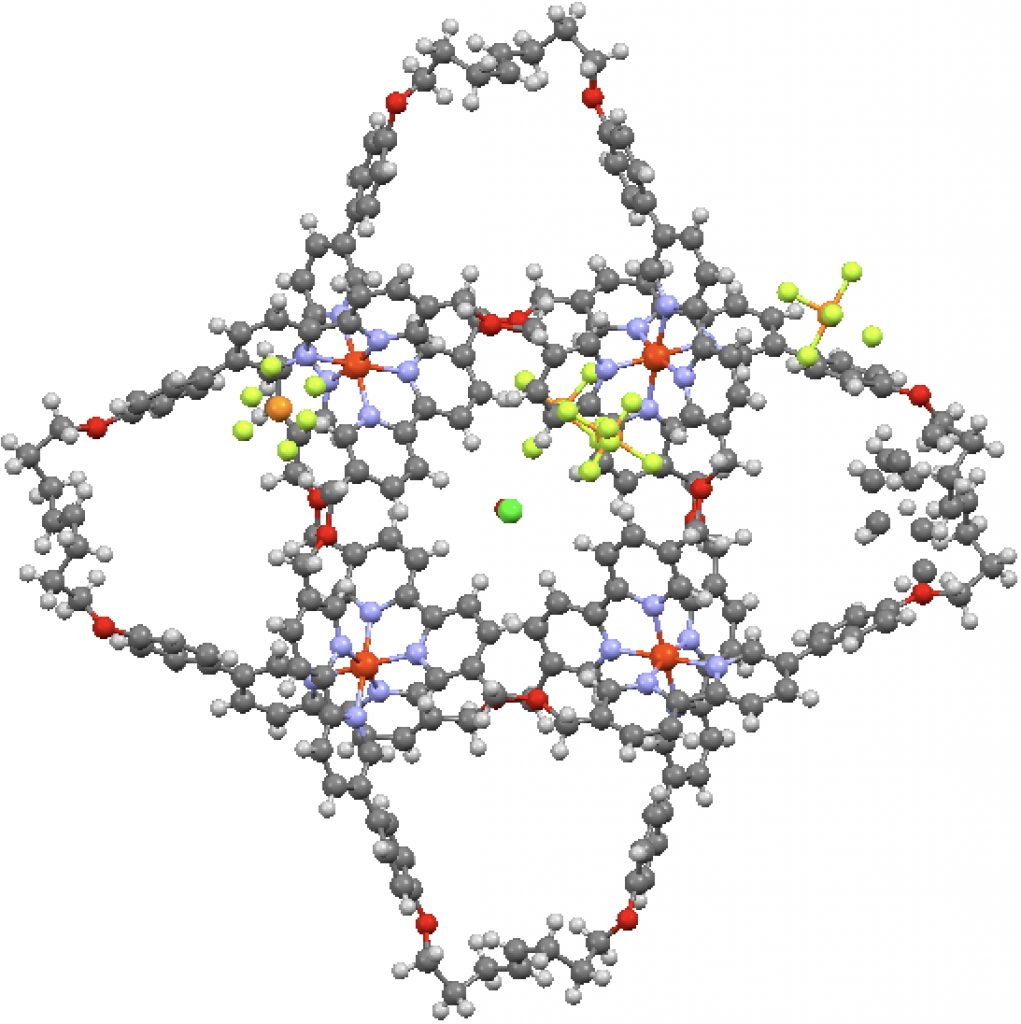
This is one of those posts of a molecule whose very structure is interesting enough to merit a picture and a 3D model. The study reports a molecular knot with the remarkable number of eight crossings.

Here is an inside peek at another one of Derek Lowe’s 250 milestones in chemistry, the polymorphism of Ritonavir. The story in a nutshell concerns one of a pharma company’s worst nightmares;

My holiday reading has been Derek Lowe’s excellent Chemistry Book setting out 250 milestones in chemistry, organised by year. An entry for 1920 entitled hydrogen bonding seemed worth exploring in more detail here. As with many historical concepts, it can often take a few years to coalesce into something we would readily recognise today, and hydrogen bonds are no exception.
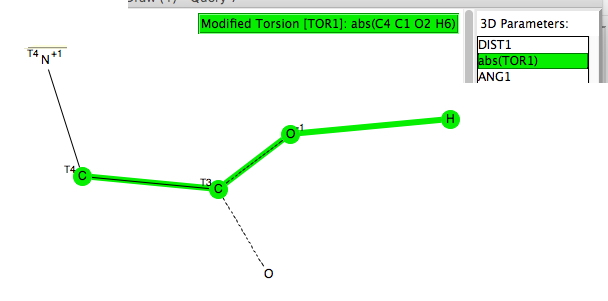
The previous posts produced discussion about the dipole moments of highly polar molecules. Here to produce some reference points for further discussion I look at the dipole moment of glycine, the classic zwitterion (an internal ion-pair).
A project fork is defined (in computing) as creating a distinct and separate strand from an existing (coding) project. Here I apply the principle to the polar azulene 4 explored in an earlier post, taking m-benzyne as a lower homologue of azulene as my starting point.
I am completing my survey of the vote for molecule of the year candidates, which this year seems focused on chemical records of one type or another.