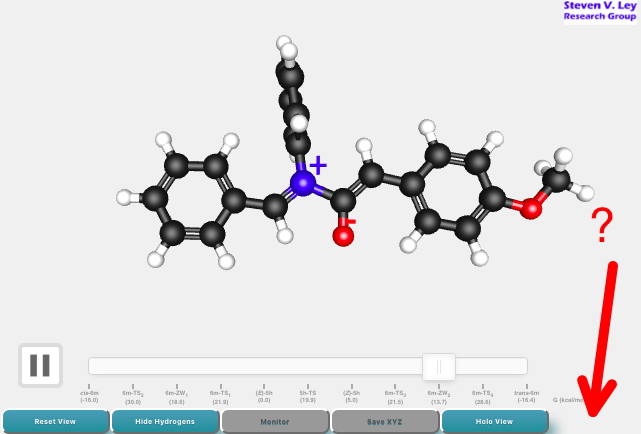
Bromoallene is a pretty simple molecule, with two non-equivalent double bonds. How might it react with an electrophile, say dimethyldioxirane (DMDO) to form an epoxide? Here I explore the difference between two different and very simple approaches to predicting its reactivity.
In the previous post, I noted that a chemistry publisher is about to repeat an earlier experiment in serving pre-prints of journal articles.
This week the ACS announced its intention to establish a “ChemRxiv preprint server to promote early research sharing“. This was first tried quite a few years ago, following the example of especially the physicists. As I recollect the experiment lasted about a year, attracted few submissions and even fewer of high quality.
The previous post contained an exploration of the anomeric effect as it occurs at an atom centre X for which the effect is manifest in crystal structures.
In the last few posts, I have explored the anomeric effect as it occurs at an atom centre X. Here I try to summarise the atoms for which the effect is manifest in crystal structures.
Here is a little molecule that can be said to be pretty electron rich. There are lots of lone pairs present, and not a few electron-deficient σ-bonds. I thought it might be fun to look at the stereoelectronic interactions set up in this little system.
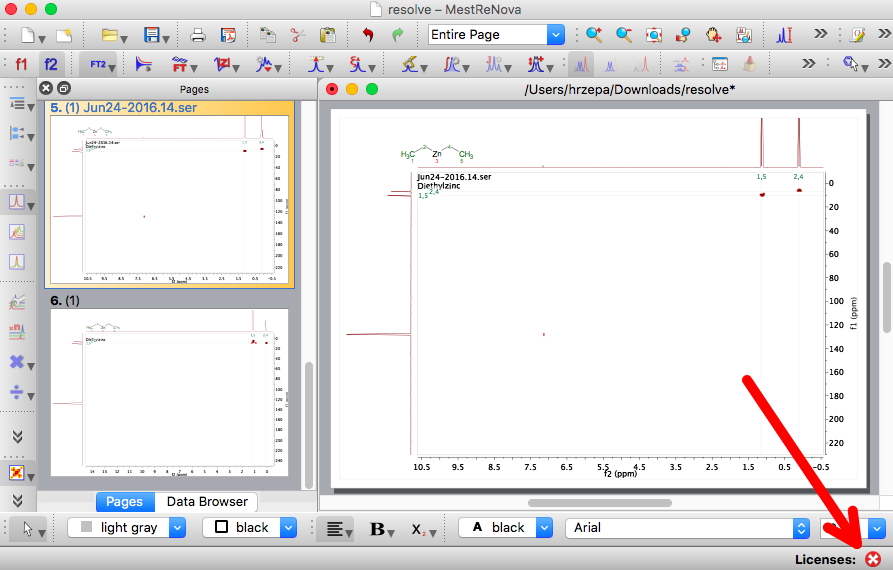
In March, I posted from the ACS meeting in San Diego on the topic of Research data: Managing spectroscopy-NMR, and noted a talk by MestreLab Research on how a tool called Mpublish in the forthcoming release of their NMR analysis software Mestrenova could help. With that release now out, the opportunity arose to test the system.
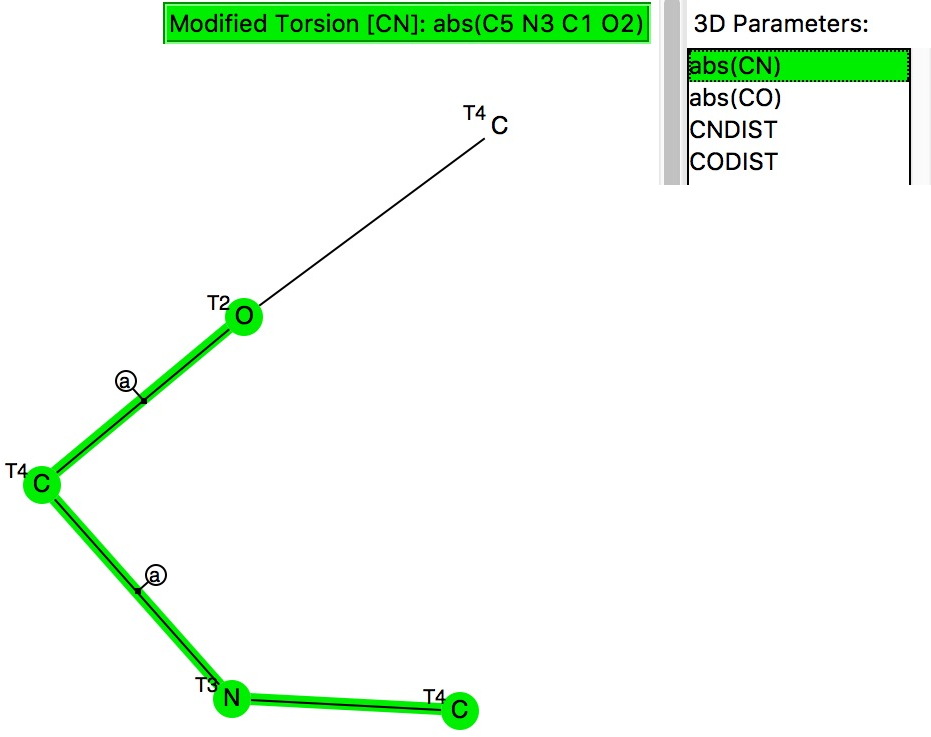
The previous post looked at anomeric effects set up on centres such as B, Si or P, and involving two oxygen groups attached to these atoms.
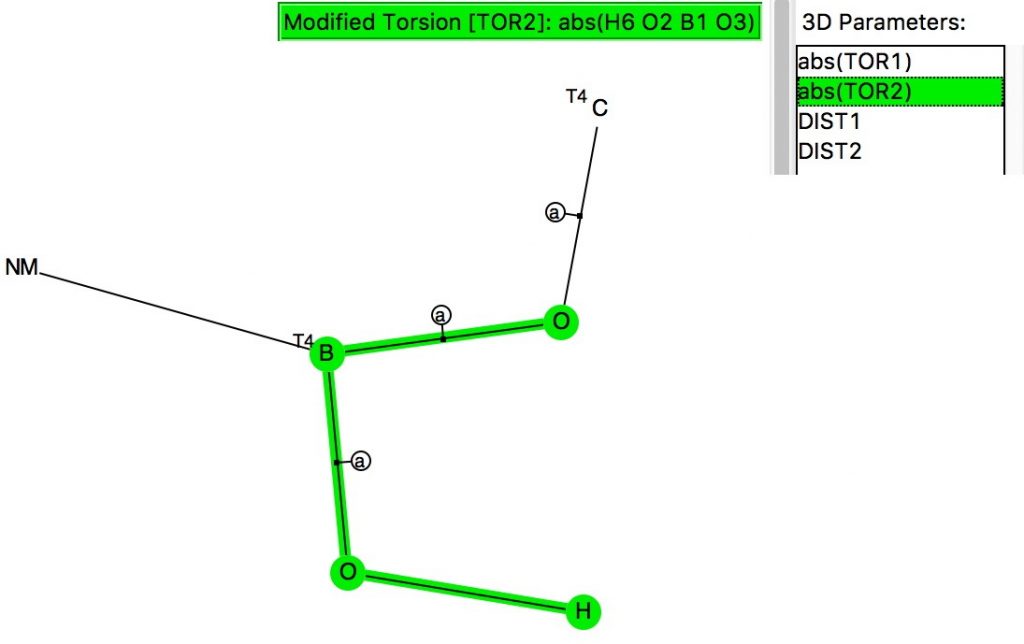
The anomeric effect occurs at 4-coordinate (sp 3 ) carbon centres carrying two oxygen substituents and involves an alignment of a lone electron pair on one oxygen with the adjacent C-O σ*-bond of the other oxygen. Here I explore whether other centres can exhibit the phenomenon.
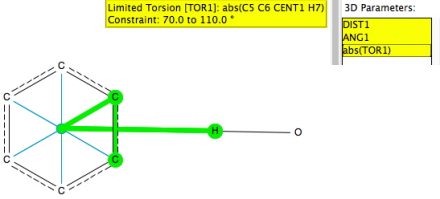
I previously used data mining of crystal structures to explore the directing influence of substituents on aromatic and heteroaromatic rings. Here I explore, quite literally, a different angle to the hydrogen bonding interactions between a benzene ring and OH or NH groups. I start by defining a benzene ring with a centroid.
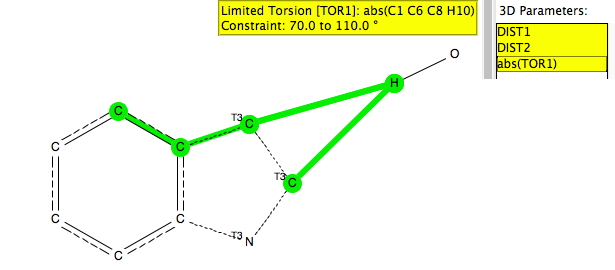
This is a follow-up to the post on exploring the directing influence of (electron donating) substituents on benzene[cite]10.1021/acs.jchemed.5b00346[/cite] with the focus on heteroaromatic rings such indoles, pyrroles and group 16 analogues (furans, thiophenes etc). The search query is shown above (and is available here[cite]10.14469/hpc/665[/cite]). As before, the distance is compared from an electrophile, modelled as