The autoionization of water involves two molecules transfering a proton to give hydronium hydroxide, a process for which the free energy of reaction is well known. Here I ask what might happen with the next element along in the periodic table, F. I have been unable to find much about the autoionization of HF in the literature;
Earlier, I constructed a possible model of hydronium hydroxide, or H 3 O + .OH^– ^One way of assessing the quality of the model is to calculate the free energy difference between it and two normal water molecules and compare the result to the measured difference. Here I apply a further test of the model using isotopes.
I want to describe a recent attempt by a group of collaborators to share the research data associated with their just published article.[cite]10.1021/jacs.5b13070[/cite] I am here introducing things in a hierarchical form (i.e. not necessarily the serial order in which actions were taken). The data repository selected for the data sharing is described by (m3data) doi: 10.17616/R3K64N[cite]10.17616/R3K64N[/cite] A collaborative
Scientists are familiar with the term data, at least in a scientific or chemical context, but appreciating metadata (meaning "after", or "beyond") is slightly more subtle, in the sense of using it to mean data about data. The challenge lies in clarifying where the boundary between data and its metadata lies and in specifying and controlling the vocabulary used for these metadata descriptions.
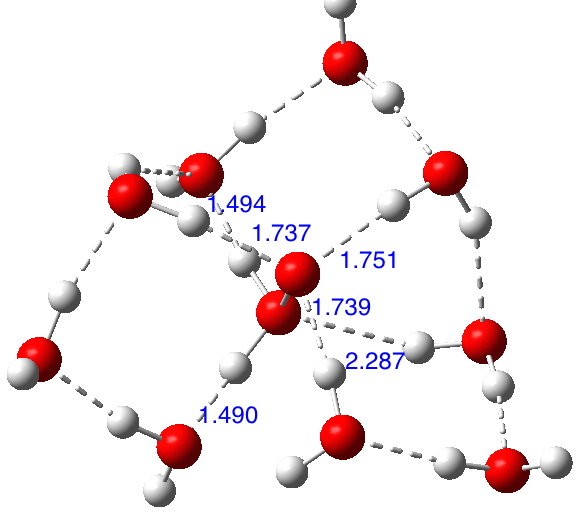
If H 3 N + -O – is viable compared with its tautomer H 2 N-OH when carrying water bridges, then why not try H 2 O + -O – vs HO-OH? There are no examples to be found in crystal structures! The solvated structure of H 2 O + -O – is modified directly from that of H 3 N + -O – and
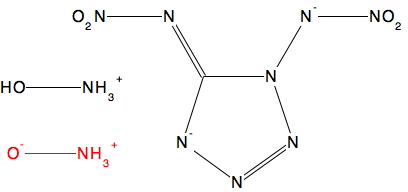
In the previous post I described how hydronium hydroxide or H 3 O + …HO – , an intermolecular tautomer of water, has recently been observed captured inside an organic cage[cite]10.1002/chem.201406383[/cite] and how the free-standing species in water can be captured computationally with the help of solvating water bridges.
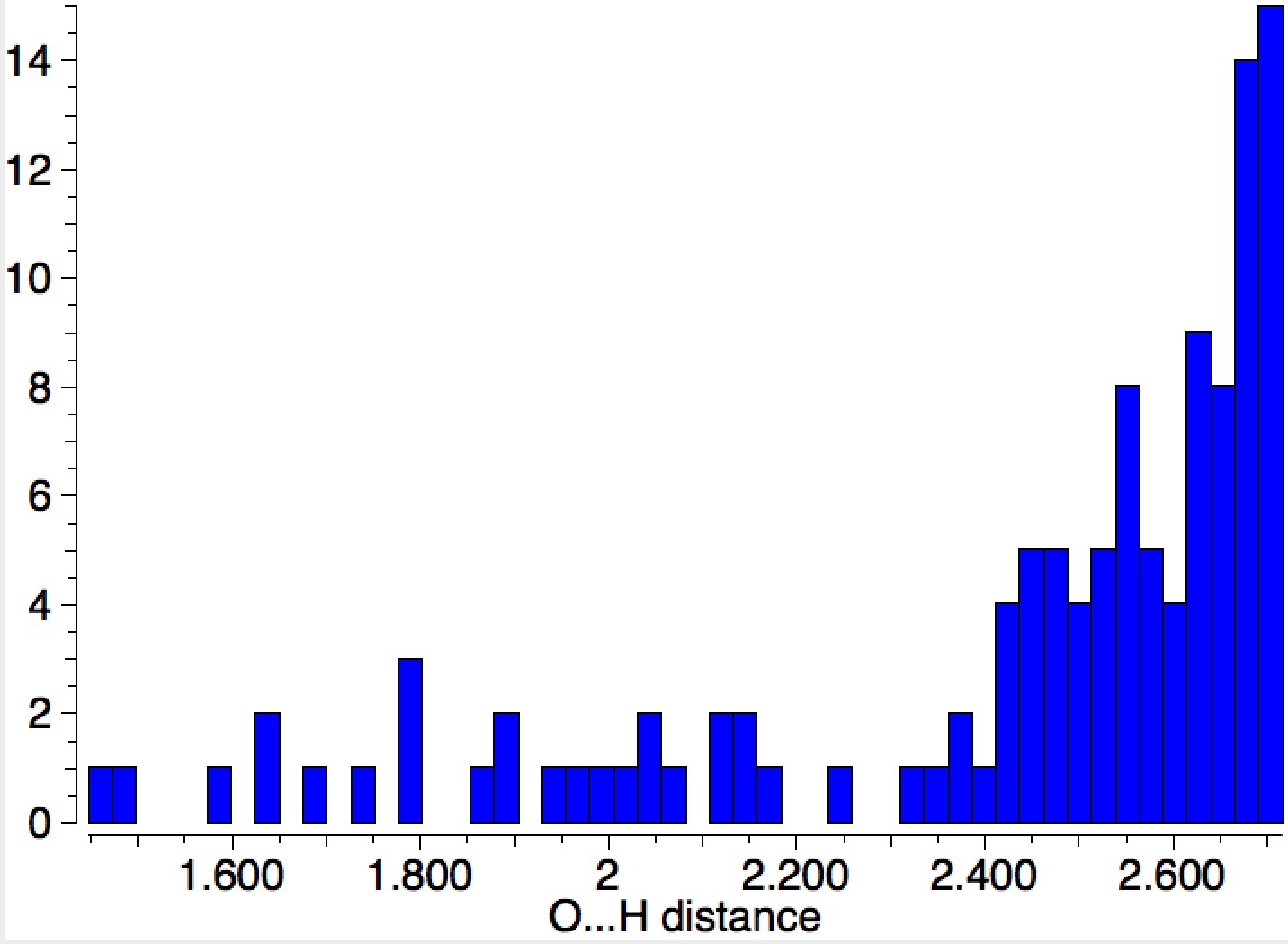
Ammonium hydroxide (NH 4 + …OH – ) can be characterised quantum mechanically when stabilised by water bridges connecting the ion-pairs.
Publishing embargoes seem a relatively new phenomenon, probably starting in areas of science when the data produced for a scientific article was considered more valuable than the narrative of that article. However, the concept of the embargo seems to be spreading to cover other aspects of publishing, and I came across one recently which appears to take such embargoes into new and uncharted territory.
Previously, I looked at models of how ammonia could be protonated by water to form ammonium hydroxide.
A celebration of the life and work of the great chemist Paul von R. Schleyer was held this week in Erlangen, Germany. There were many fantastic talks given by some great chemists describing fascinating chemistry. Here I highlight the presentation given by Andy Streitwieser on the topic of organolithium chemistry, also a great interest of Schleyer's over the years.
Augmented reality, a superset if you like of virtual reality (VR), has really been hitting the headlines recently. Like 3D TV, its been a long time coming! Since ~1994 or earlier, there have been explorations of how molecular models can be transferred from actual reality to virtual reality using conventional computers (as opposed to highly specialised ones). It was around then that a combination of software (Rasmol) and