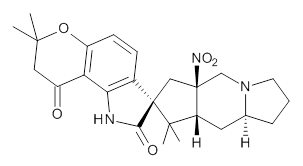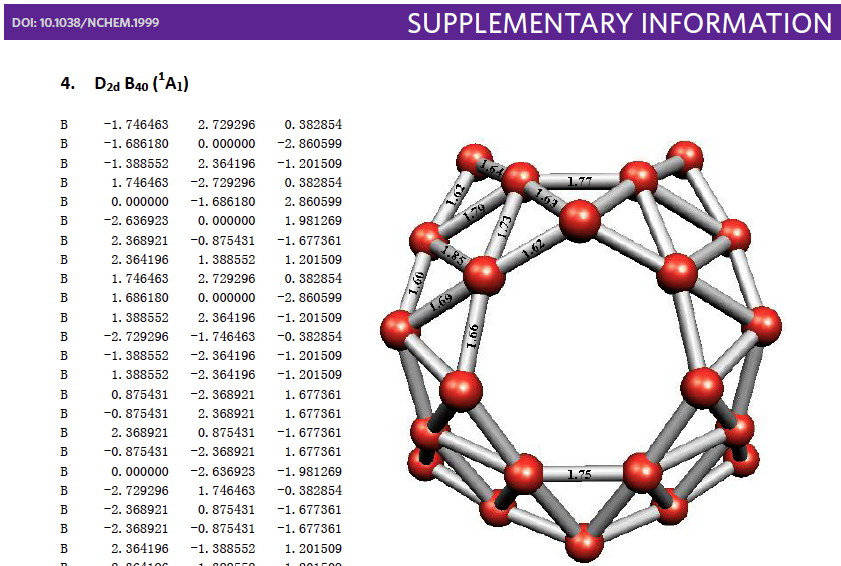
Whilst clusters of carbon atoms are well-known, my eye was caught by a recent article describing the detection of a cluster of boron atoms, B 40 to be specific.[cite]10.1038/nchem.1999[/cite] My interest was in how the σ and π-electrons were partitioned. In a C 40 , one can reliably predict that each carbon would contribute precisely one π-electron. But boron, being more electropositive, does not always play like that.