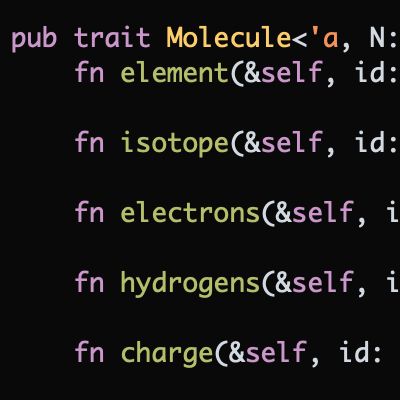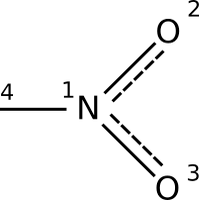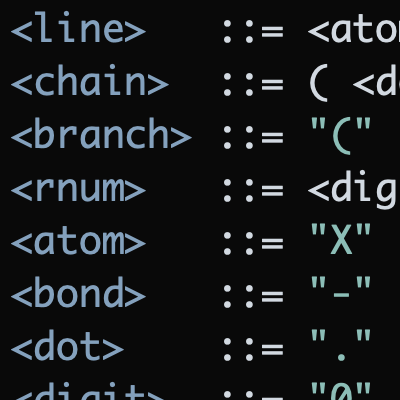
Hydrogen bears the distinction of being both the most common element in the universe and one of the most predicable elements on the periodic table. Outnumbering carbon in many organic molecules, hydrogen rarely participates in anything more exciting than hydrogen bonding or acid-base reactions. For the most part, the monovalent hydrogen atoms studding the average organic molecule are ignored.