I have had some interesting discussions recently regarding metadata. What emerges is that it can be quite a broadly defined concept and it is clear that a variety of answers might be obtained when asking the simple question “what is it useful for?” Here I set out some of my answers to that question.
The World-Wide-Web is currently celebrating its 30th anniversary; you can get the T-shirt in the CERN visitor centre! Five years on, in May 1994, the first Web conference took place (WWW94) at CERN and now celebrating its own 25th anniversary.
In August 2016, the launch of a chemistry pre-print service ChemRxiv was announced. I was phoned a day or so later by a staff journalist at C&E News for my opinion.
This is a follow up to my earlier post about C⩸N+, itself inspired by this ChemRxiv pre-print which describes a chemical synthesis of singlet biradicaloid C2 and its proposed identification as such by chemical trapping.
Although the small diatomic molecule known as dicarbon or C2 has been known for a long time, its properties and reactivity have really only been determined via its very high temperature generation. My interest started in 2010, when I speculatively proposed here that the related isoelectronic species C⩸N+ might sustain a quadruple bond.
Ken Houk’s group has recently published this study of cycloaddition reactions, using a combination of classical transition state location followed by molecular dynamics trajectory calculations, and to which Steve Bachrach’s blog alerted me. The reaction struck me as being quite polar (with cyano groups) and so I took a look at the article to see […]
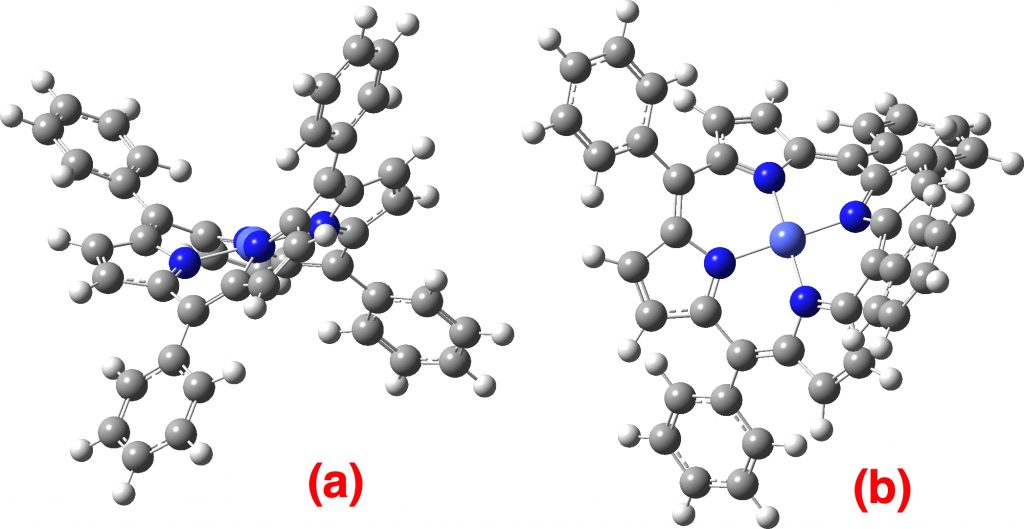
Previously, I explored (computationally) the normal vibrational modes of Co(II)-tetraphenylporphyrin (CoTPP) as a “flattened” species on copper or gold surfaces for comparison with those recently imaged. The initial intent was to estimate the “flattening” energy. There are six electronic possibilities for this molecule on a metal surface.
In a previous post, I looked at the Findability of FAIR data in common chemistry journals. Here I move on to the next letter, the A = Accessible.
The topic of this post originates from a recent article which is attracting much attention. The technique uses confined light to both increase the spatial resolution by around three orders of magnitude and also to amplify the signal from individual molecules to the point it can be recorded.
In recent years, findable data has become ever more important (the F in FAIR). Here I test that F using the DataCite search service.
The conventional procedures for reporting analysis or new results in science is to compose an “article”, augment that perhaps with “supporting information” or “SI”, submit to a journal which undertakes peer review, with revision as necessary for acceptance and finally publication.