I started this story by looking at octet expansion and hypervalence in non-polar hypercoordinate species such as S(-CH3)6, then moved on to S(=CH2)3. Finally now its the turn of S(≡CH)2.‡
Previously: “Non-polar” species such as SeMe 6 , SMe 6 , ClMe 3 , ClMe 5 all revealed interesting properties for the Se-C, S-C or Cl-C “single” bonds. The latter two examples in particular hinted at internal structures for these single bonds, as manifested by two ELF basins for some of the bonds.
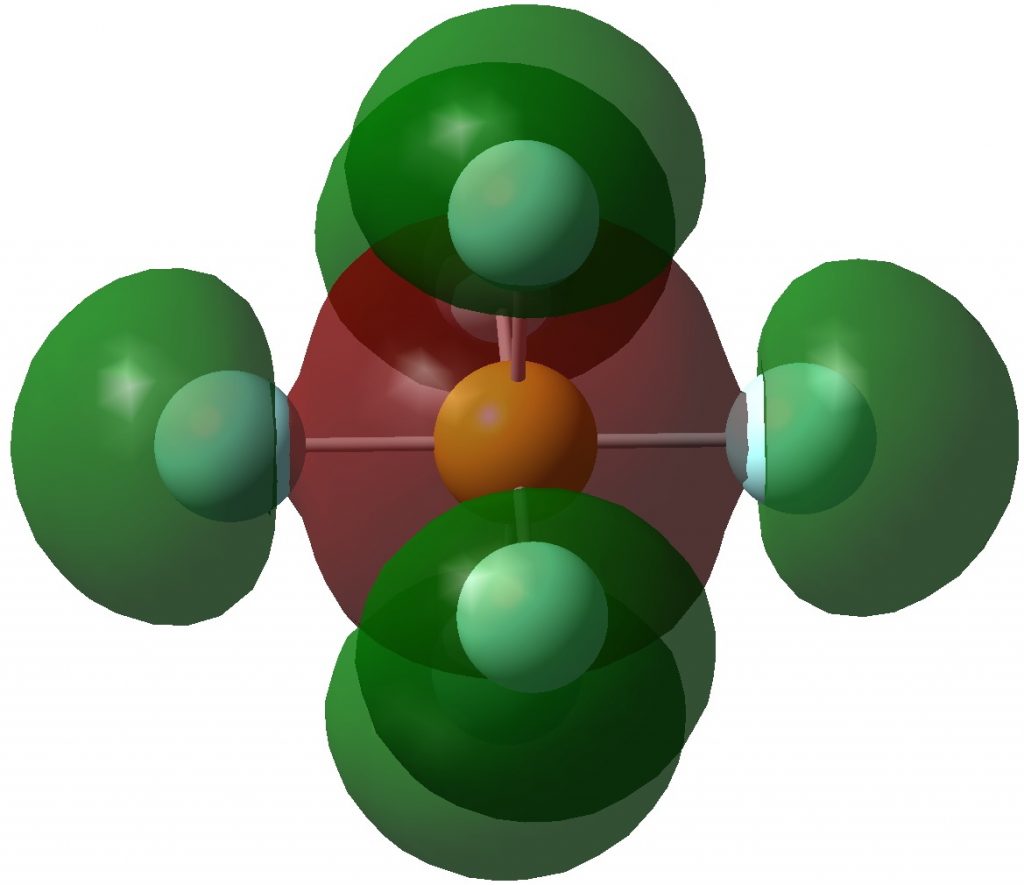
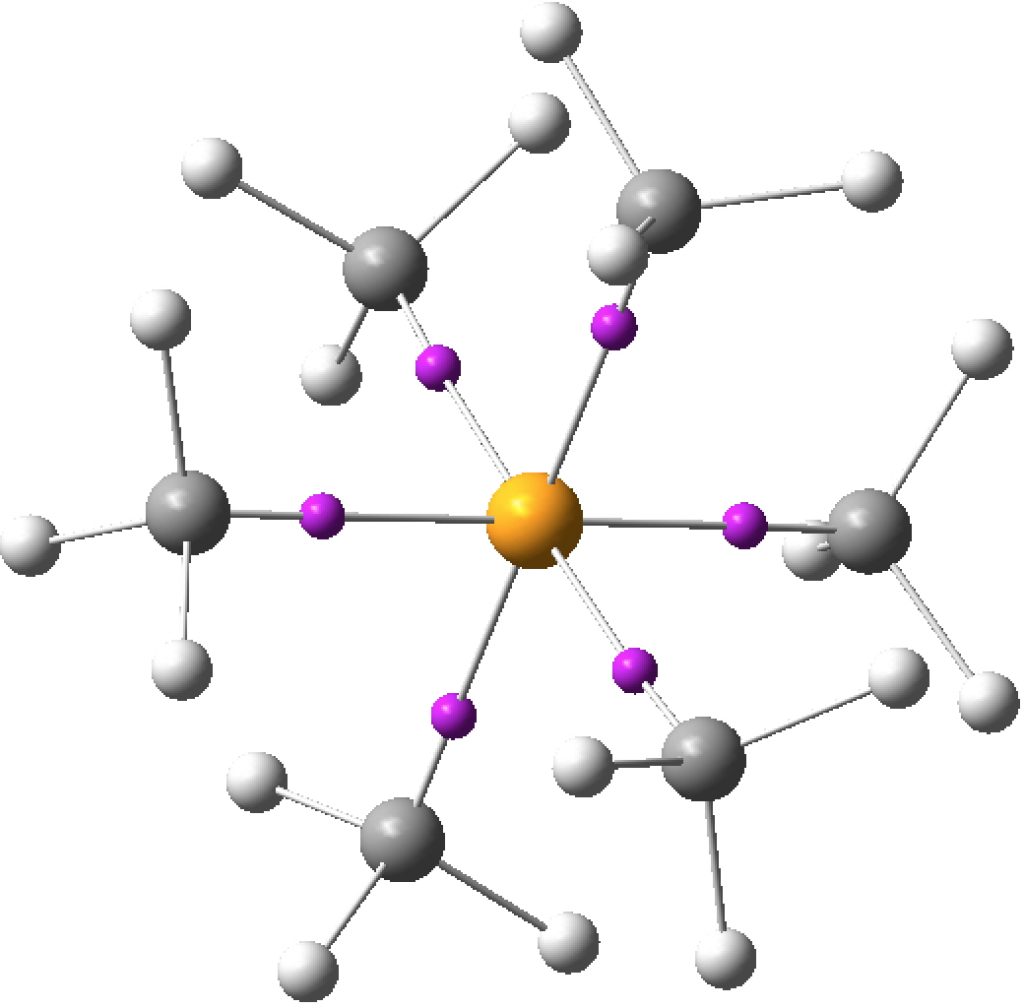
Following on from discussing octet expansion in species such as SeMe6, ClMe3 and ClMe5, I felt impelled to return to SF6, often used as an icon for hypervalence.
PIDapalooza is a new forum concerned with discussing all things persistent, hence PID. You might wonder what possible interest a chemist might have in such an apparently arcane subject, but think of it in terms of how to find the proverbial needle in a haystack in a time when needles might look all very similar.
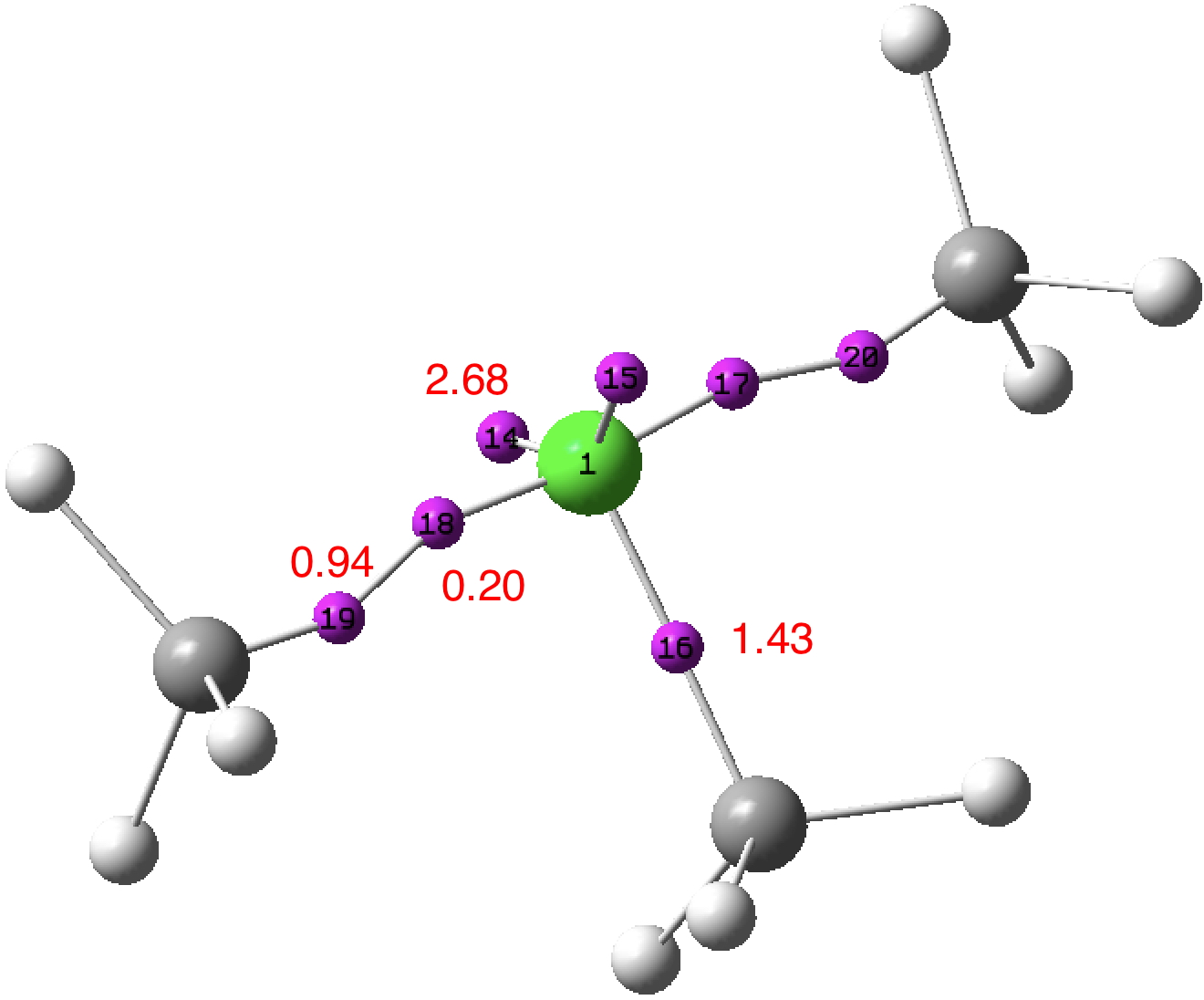
A few years back, I took a look at the valence-shell electron pair repulsion approach to the geometry of chlorine trifluoride, ClF3 using so-called ELF basins to locate centroids for both the covalent F-Cl bond electrons and the chlorine lone-pair electrons.
One thread that runs through this blog is that of hypervalency . It was therefore nice to come across a recent review of the concept[1] which revisits the topic, and where a helpful summary is given of the evolving meanings over time of the term hypervalent.
An N-B single bond is iso-electronic to a C-C single bond, as per below. So here is a simple question: what form does the distribution of the lengths of these two bonds take, as obtained from crystal structures?
We have heard a lot about OA or Open Access (of journal articles) in the last five years, often in association with the APC (Article Processing Charge) model of funding such OA availability. Rather less discussed is how the model of the peer review of these articles might also evolve into an Open environment.
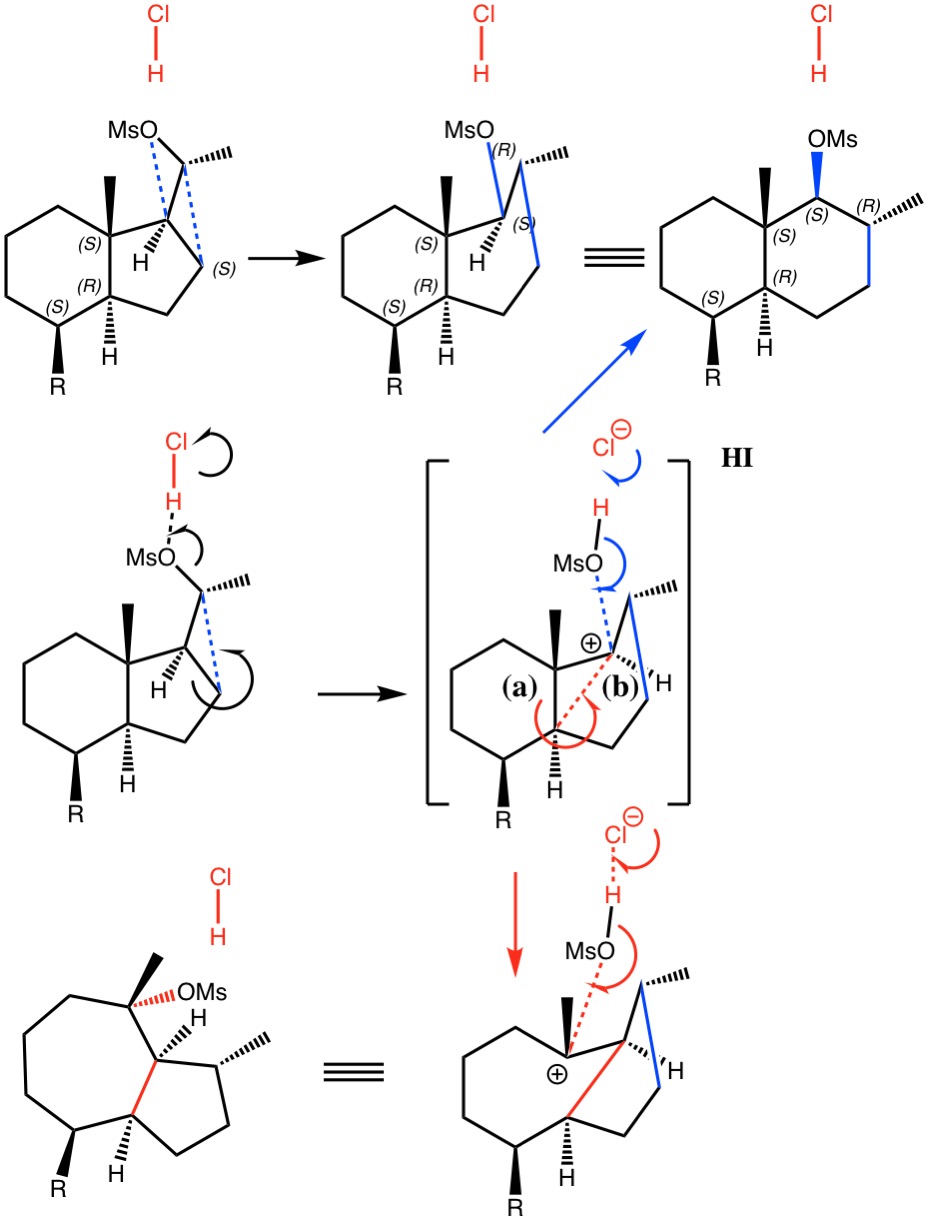
I noted in my WATOC conference report a presentation describing the use of calculated reaction barriers (and derived rate constants) as mechanistic reality checks. Computations, it was claimed, have now reached a level of accuracy whereby a barrier calculated as being 6 kcal/mol too high can start ringing mechanistic alarm bells.
A recent article reports, amongst other topics, a computationally modelled reaction involving the capture of molecular hydrogen using a substituted borane (X=N, Y=C). The mechanism involves an initial equilibrium between React and Int1, followed by capture of the hydrogen by Int1 to form a 5-coordinate borane intermediate (Int2 below, as per Figure 11).‡ This was […]

Early in 2011, I wrote about how the diatomic molecule Be2 might be persuaded to improve upon its normal unbound state (bond order ~zero) by a double electronic excitation to a strongly bound species. I yesterday updated this post with further suggestions and one of these inspired this follow-up.