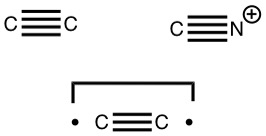
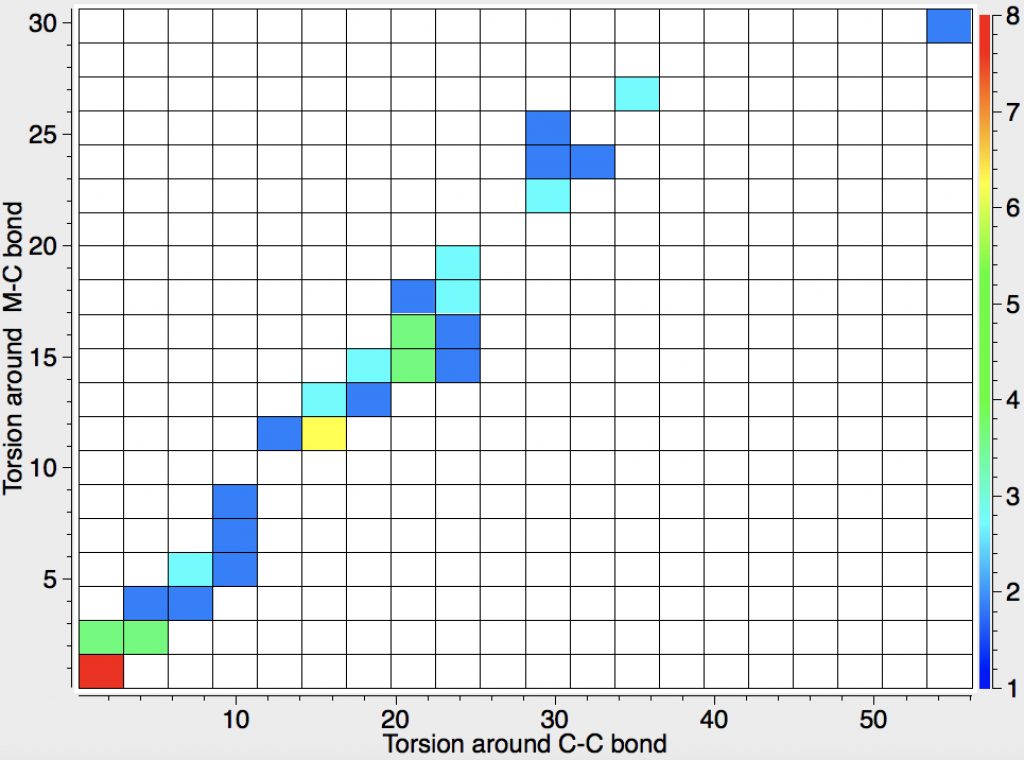
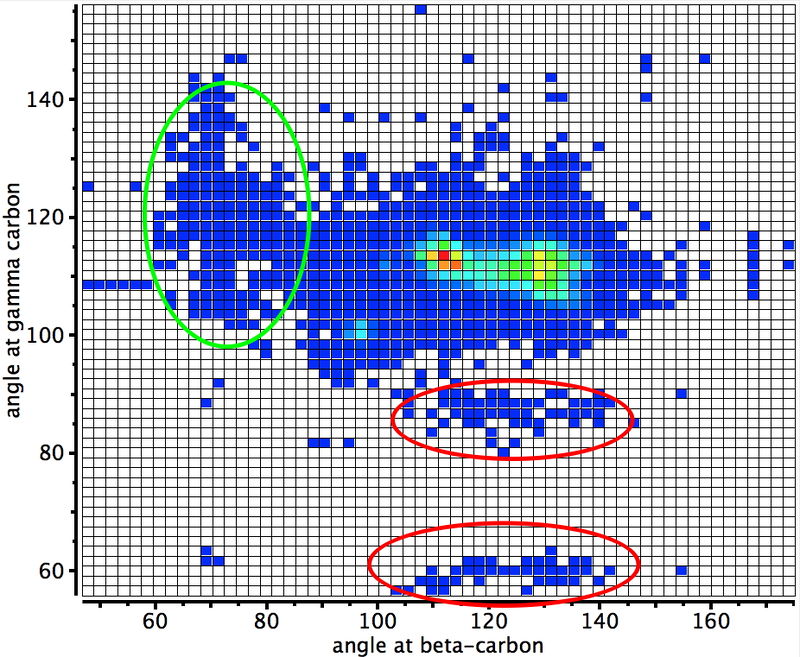
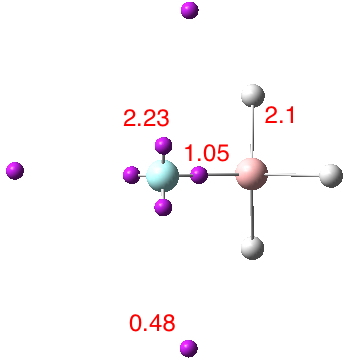
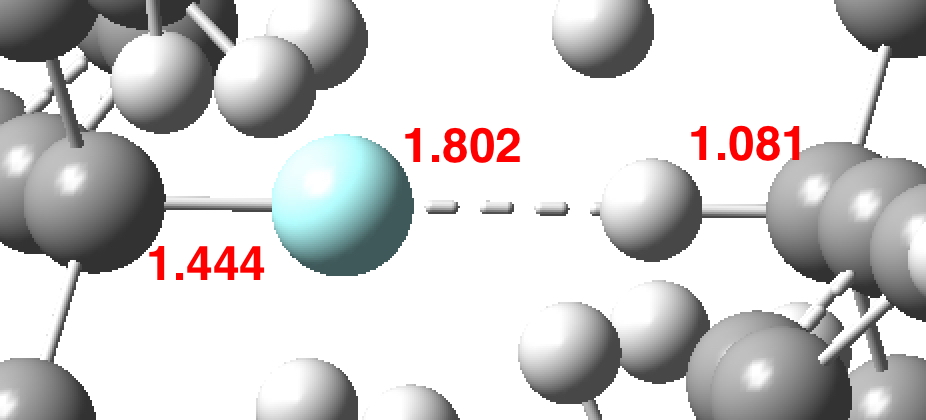
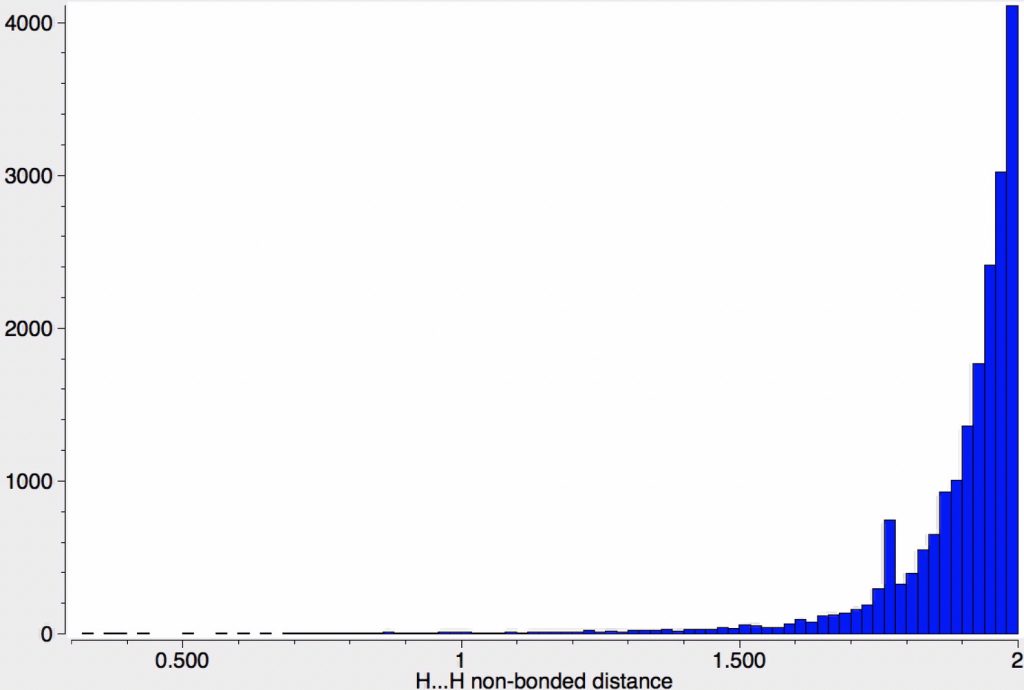
Early in 2011, I wrote about how the diatomic molecule Be2 might be persuaded to improve upon its normal unbound state (bond order ~zero) by a double electronic excitation to a strongly bound species. I yesterday updated this post with further suggestions and one of these inspired this follow-up.