
It is a sign of the times that one travels to a conference well-connected. By which I mean email is on a constant drip-feed, with venue organisers ensuring each delegate receives their WiFi password even before their room key.

It is a sign of the times that one travels to a conference well-connected. By which I mean email is on a constant drip-feed, with venue organisers ensuring each delegate receives their WiFi password even before their room key.

This is taking place in the idyllic surroundings of the Niederwald forest, Rüdesheim, Germany. Here I highlight only aspects of the first three talks.
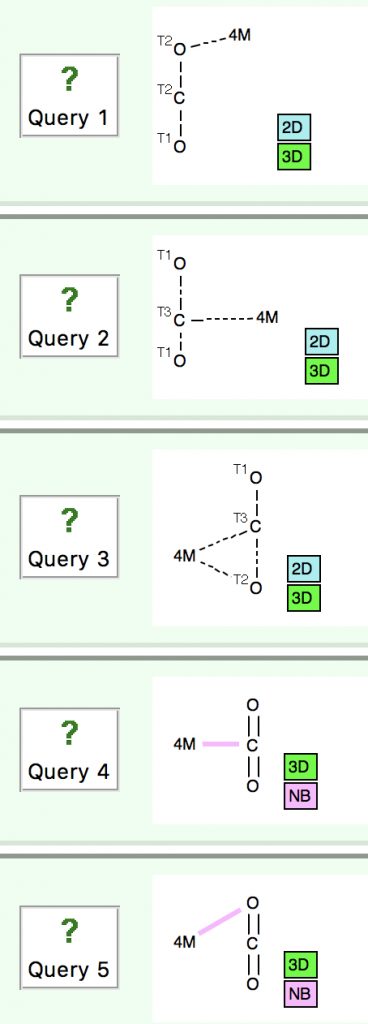
Mention carbon dioxide (CO2) to most chemists and its properties as a metal ligand are not the first aspect that springs to mind. Here thought I might take a look at how it might act as such.
Research data (and its management) is rapidly emerging as a focal point for the development of research dissemination practices.
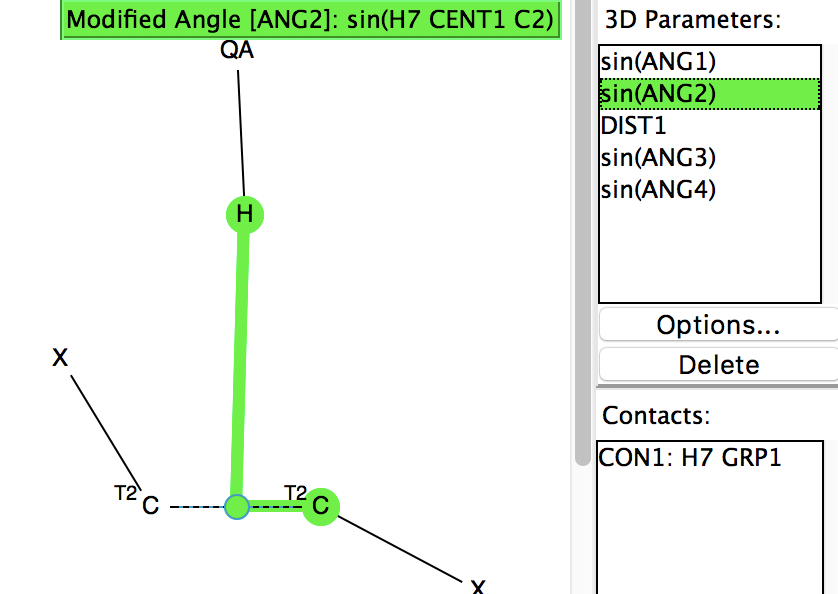
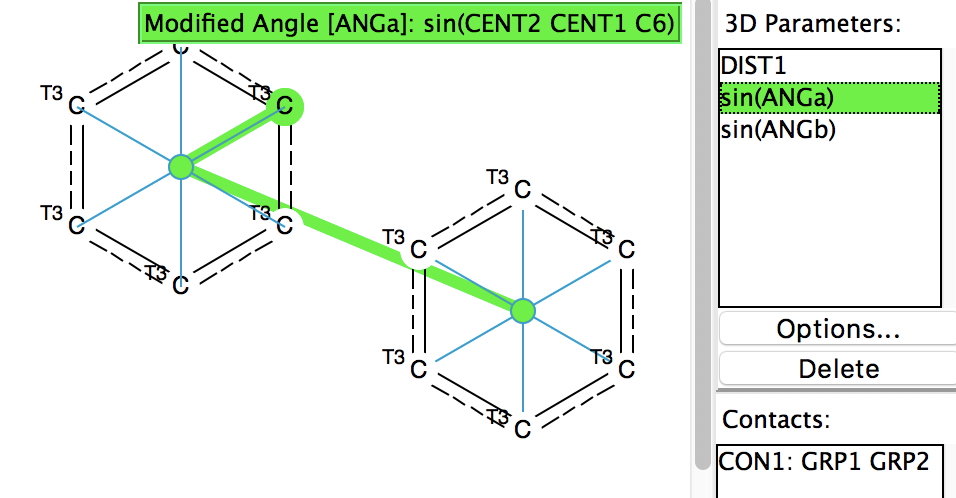
Following on from my re-investigation of close hydrogen bonding contacts to the π-face of alkenes, here now is an updated scan for H-bonds to alkynes.
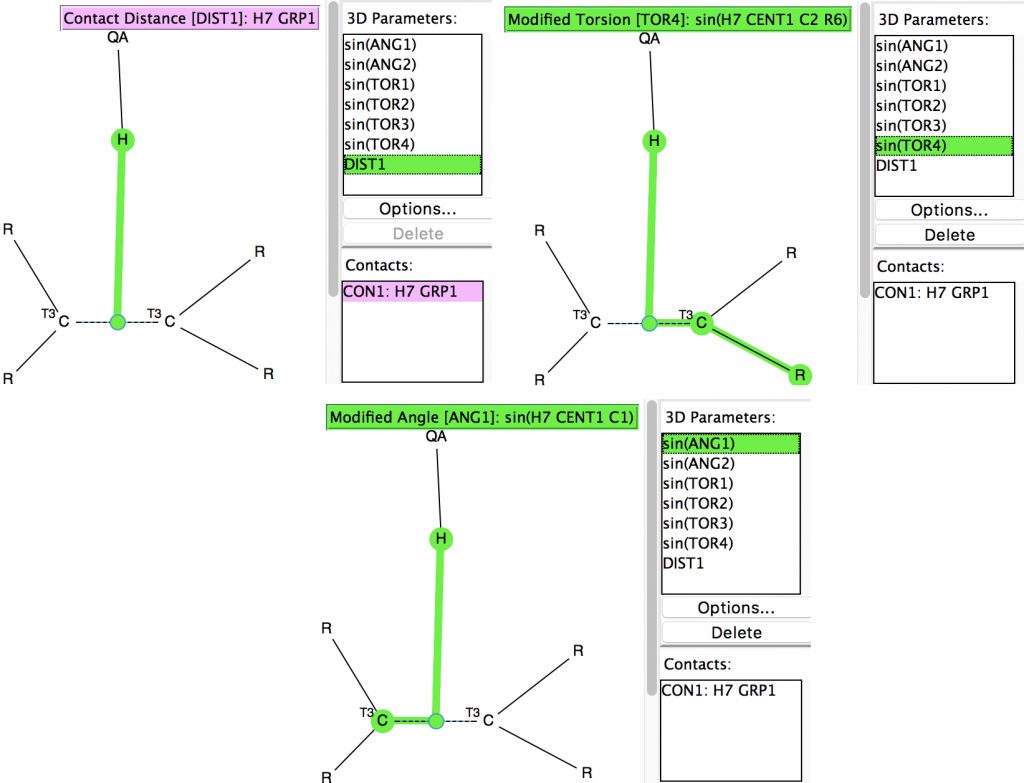
Back in the early 1990s, we first discovered the delights of searching crystal structures for unusual bonding features. One of the first cases was a search for hydrogen bonds formed to the π-faces of alkenes and alkynes. In those days the CSD database of crystal structures was a lot smaller (<80,000 structures;
Layer stacking in structures such as graphite is well-studied.
Following my conformational exploration of enols, here is one about a much more common molecule, a carboxylic acid.
Both the cyclopropenium cation and the cyclopentadienide anion are well-known 4n+2-type aromatic ions, but could the two together form an ion-pair?
Enols are simple compounds with an OH group as a substituent on a C=C double bond and with a very distinct conformational preference for the OH group. Here I take a look at this preference as revealed by crystal structures, with the theoretical explanation.
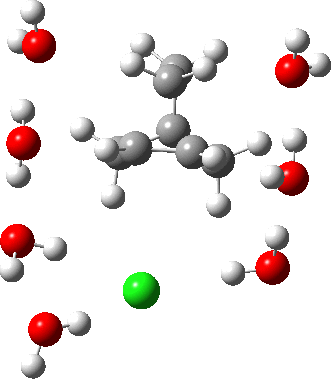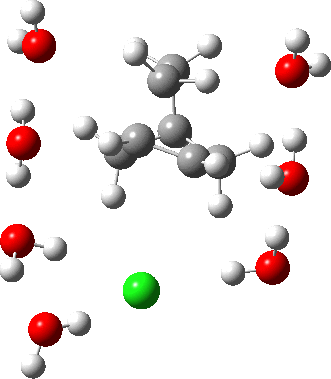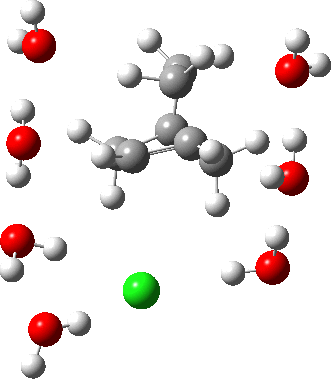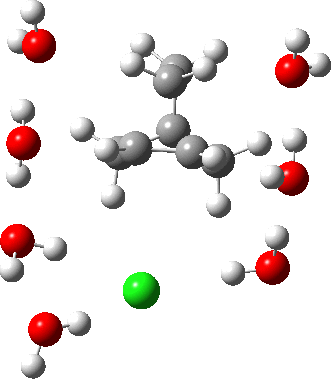
In a comment appended to an earlier post, I mused about the magnitude of the force constant relating to the interconversion between a classical and a non-classical structure for the norbornyl cation.