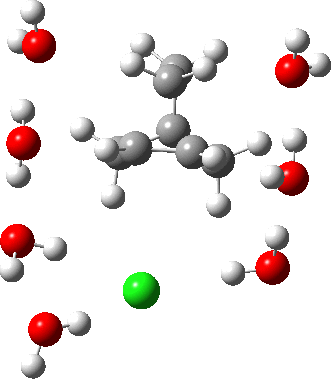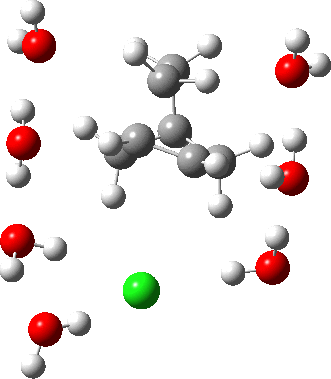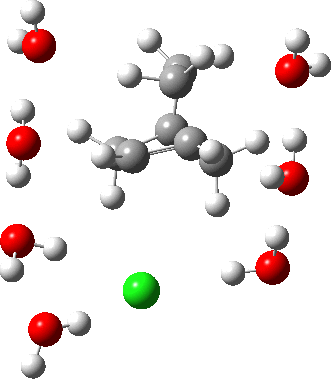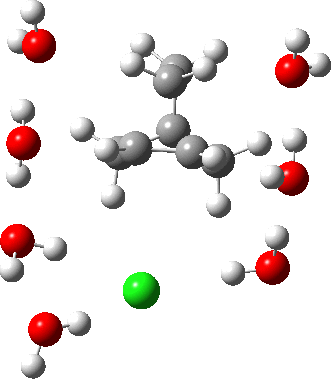
In a comment appended to an earlier post, I mused about the magnitude of the force constant relating to the interconversion between a classical and a non-classical structure for the norbornyl cation.

In a comment appended to an earlier post, I mused about the magnitude of the force constant relating to the interconversion between a classical and a non-classical structure for the norbornyl cation.
Nowadays, data supporting most publications relating to the synthesis of organic compounds is more likely than not to be found in associated “supporting information” rather than the (often page limited) article itself. For example, this article has an SI which is paginated at 907;

Occasionally one comes across a web site that manages to combine being unusual, interesting and also useful. Thus www.molinsight.net is I think a unique chemistry resource for blind and visually impaired students.
In an era when alternative facts and fake news afflict us, the provenance of scientific data becomes ever more important. Especially if that data is available as open access and exploitable by others for both valid scientific reasons but potentially also by those with other motives.
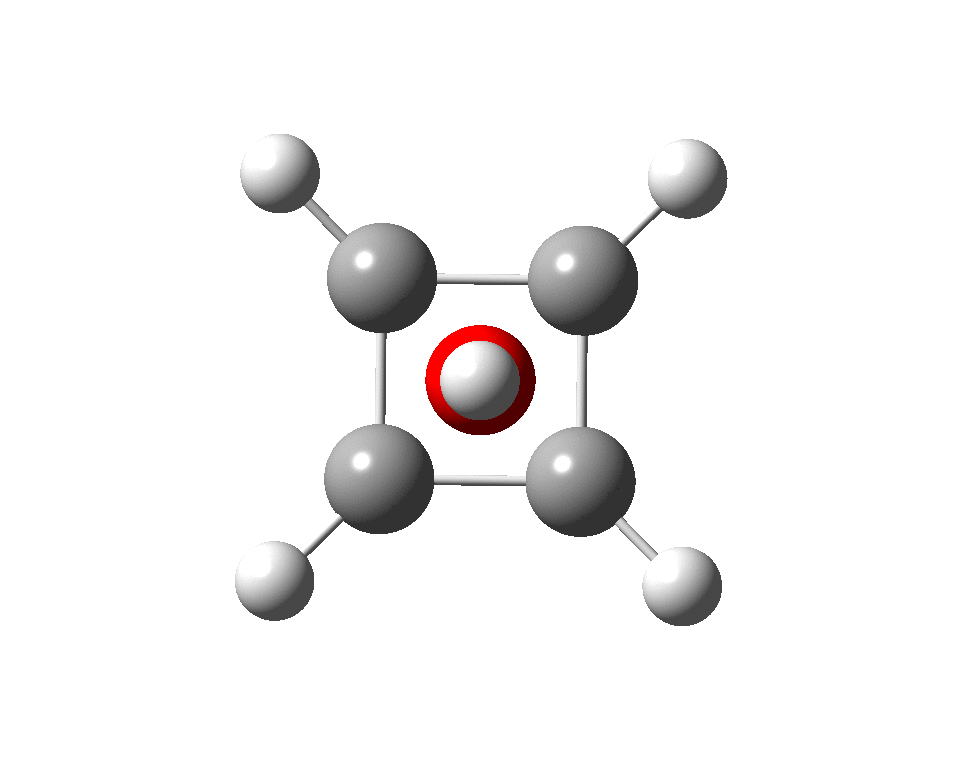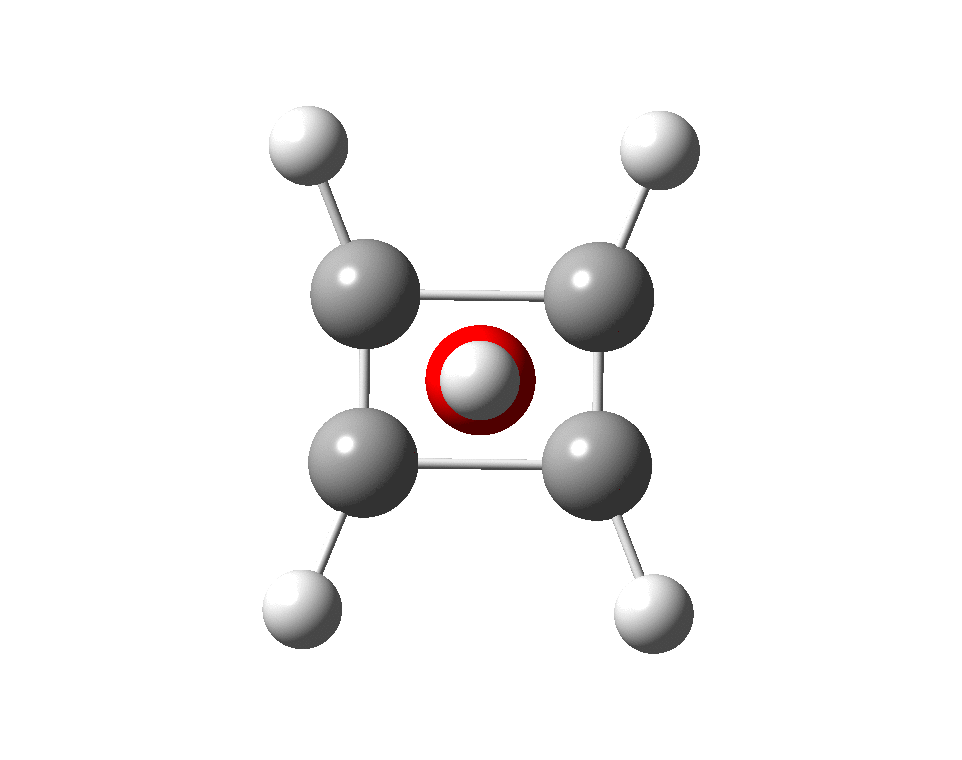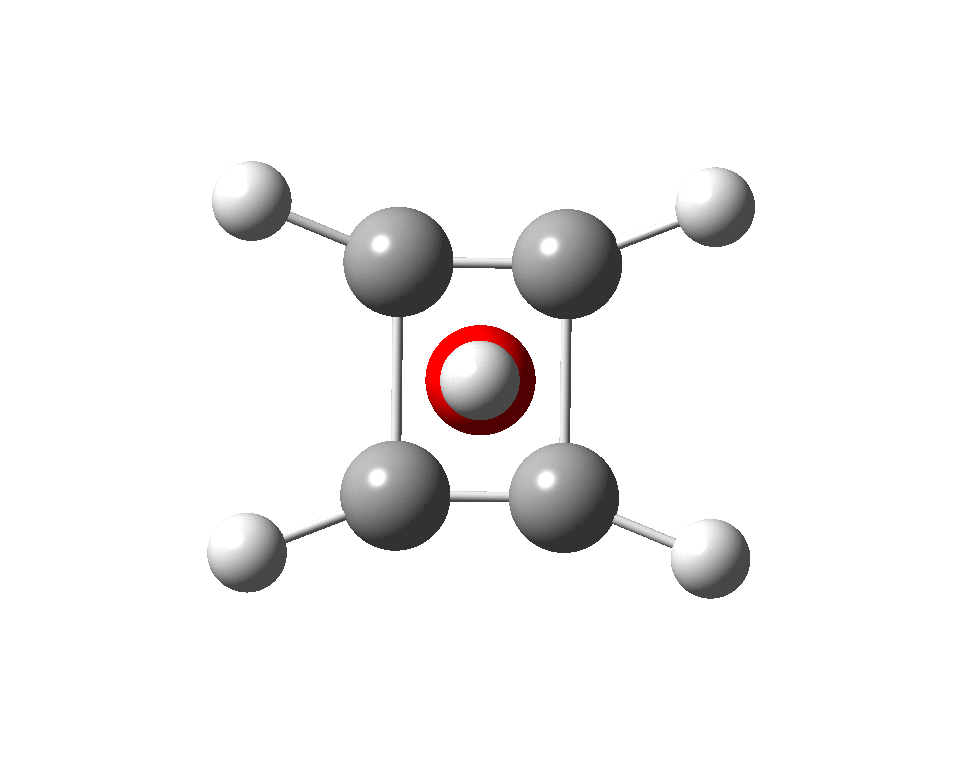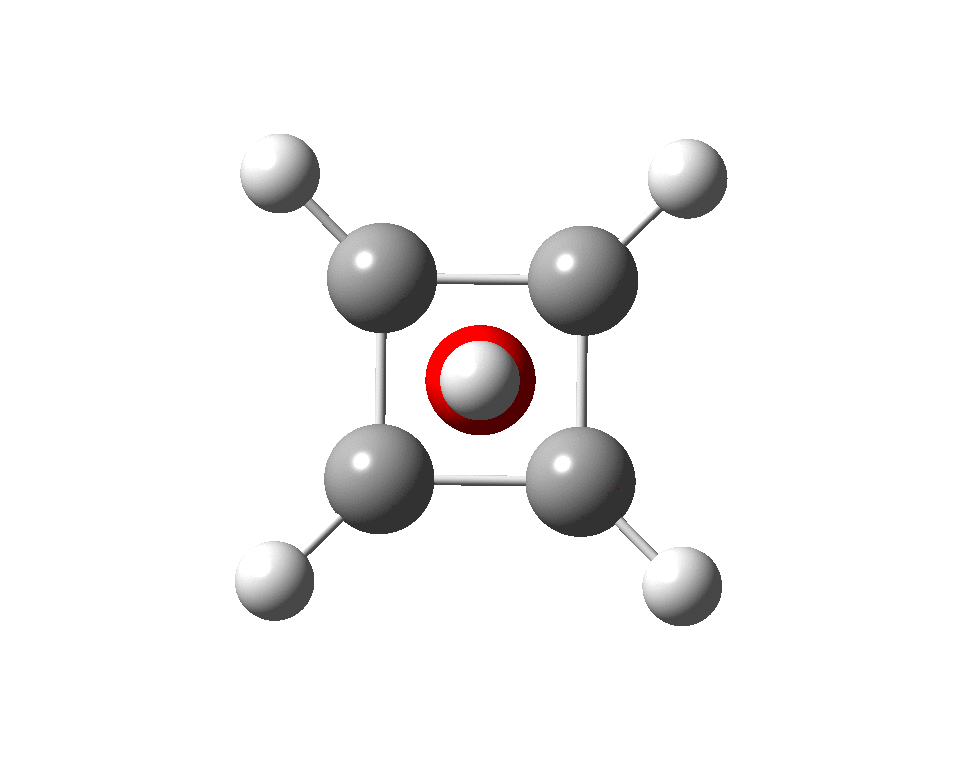
The previous post demonstrated the simple iso-electronic progression from six-coordinate carbon to five coordinate nitrogen. Here, a further progression to oxygen is investigated computationally.
A few years back I followed a train of thought here which ended with hexacoordinate carbon, then a hypothesis rather than a demonstrated reality. That reality was recently confirmed via a crystal structure, DOI:10.5517/CCDC.CSD.CC1M71QM. Here is a similar proposal for penta-coordinate nitrogen.
It is not only the non-classical norbornyl cation that has proved controversial in the past. A colleague mentioned at lunch (thanks Paul!) that tri-coordinate group 14 cations such as R3Si+ have also had an interesting history. Here I take a brief look at some of these systems.
The example a few posts back of how methane might invert its configuration by transposing two hydrogen atoms illustrated the reaction mechanism by locating a transition state and following it down in energy using an intrinsic reaction coordinate (IRC). Here I explore an alternative method based instead on computing a molecular dynamics trajectory (MD).
The Wikipedia entry on peroxydisulfate is quite short (as of today). But I suspect this article may change things..
A pyrophoric metal is one that burns spontaneously in oxygen; I came across this phenomenon as a teenager doing experiments at home.
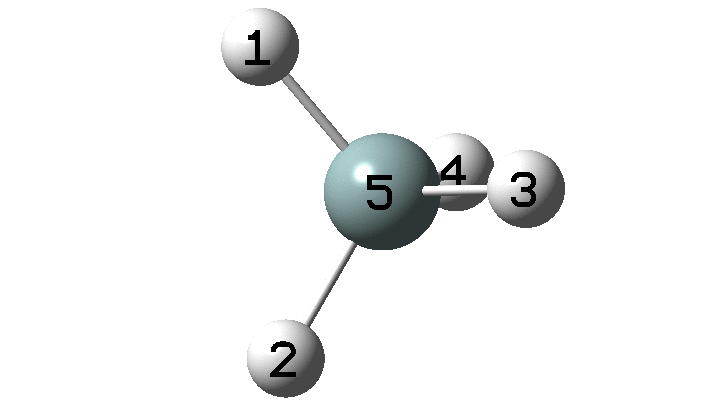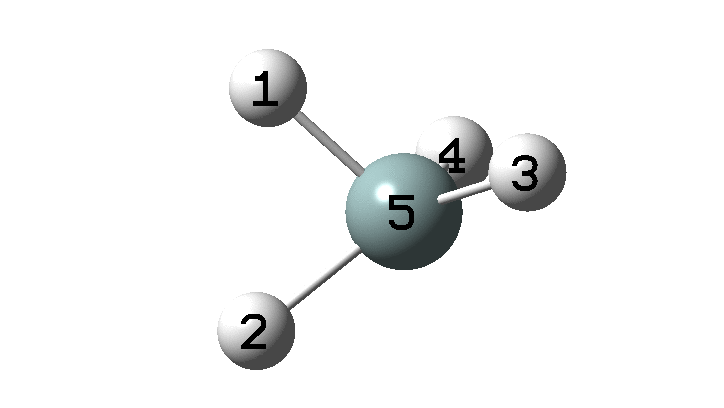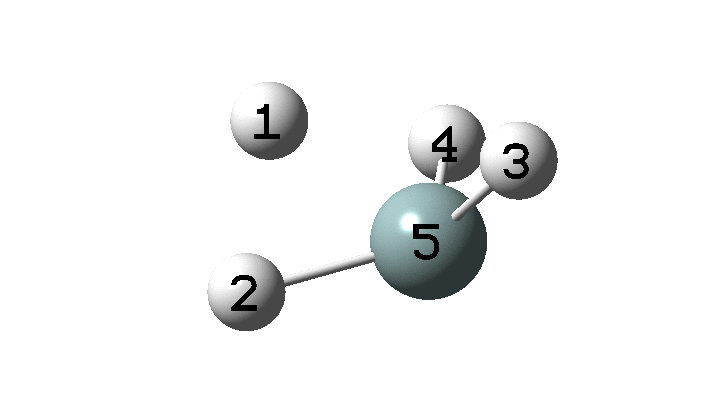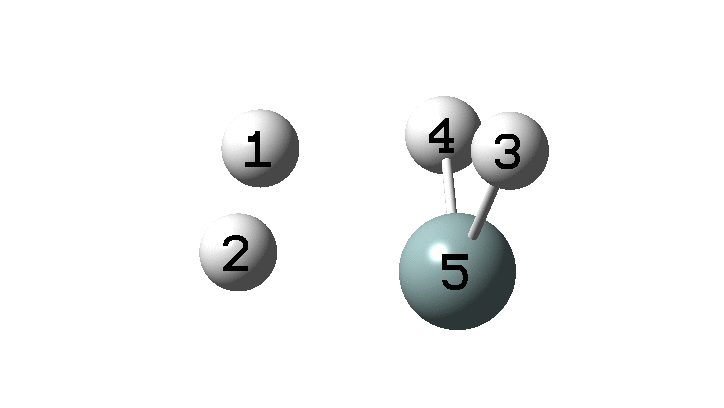
In the previous post, I found intriguing the mechanism by which methane (CH4) inverts by transposing two of its hydrogens. Here I take a look at silane, SiH4.