I am completing my survey of the vote for molecule of the year candidates, which this year seems focused on chemical records of one type or another.
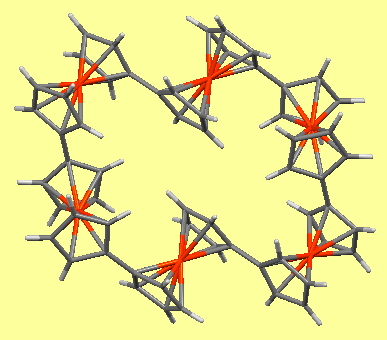
This, the fourth candidate provided by C&EN for a vote for the molecule of the year as discussed here, lays claim to the World’s most polar neutral molecule (system 1 shown below). Here I explore a strategy for extending that record.
Here is a third candidate for the C&EN “molecule of the year” vote.
Chemical and engineering news (C&EN) is asking people to vote for their molecule of the year from six highlighted candidates.
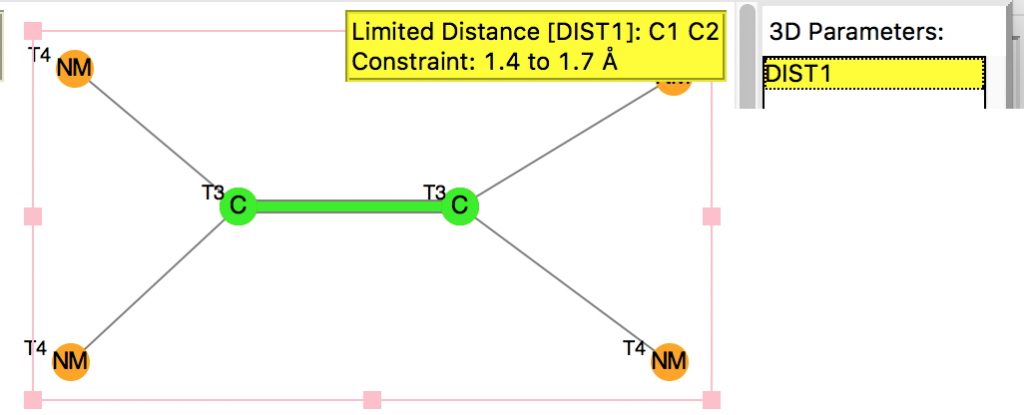
Following on from a search for long C-C bonds, here is the same repeated for C=C double bonds.
In an earlier post, I searched for small C-C-C angles, finding one example that was also accompanied by an apparently exceptionally long C-C bond (2.18Å). But this arose from highly unusual bonding giving rise not to a single bond order but one closer to one half!

Another conference, a Cambridge satellite meeting of OpenCon, and I quote here its mission: “OpenCon is a platform for the next generation to learn about Open Access, Open Education, and Open Data, develop critical skills, and catalyze action toward a more open system of research and education” targeted at students and early career academic professionals.
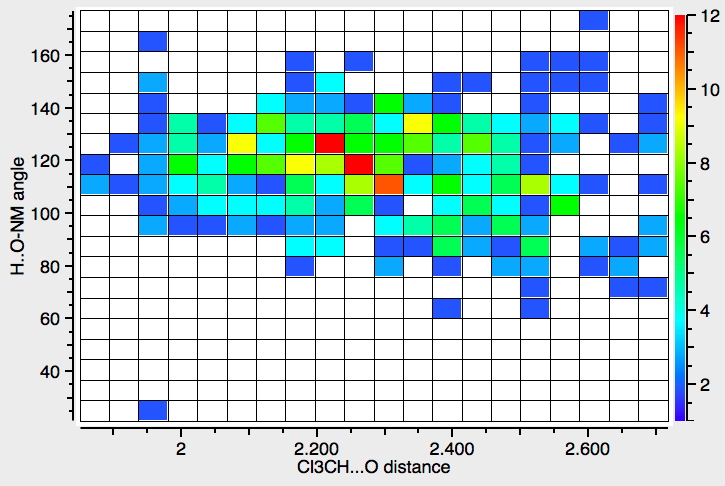
Chloroform, often in the deuterated form CDCl3, is a very common solvent for NMR and other types of spectroscopy. Quantum mechanics is increasingly used to calculate such spectra to aid assignment and the solvent is here normally simulated as a continuum rather than by explicit inclusion of one or more chloroform molecules.
This is sent from the Pidapalooza event in Reykjavik, Iceland, and is a short collection of notable things I learnt or which attracted my attention.
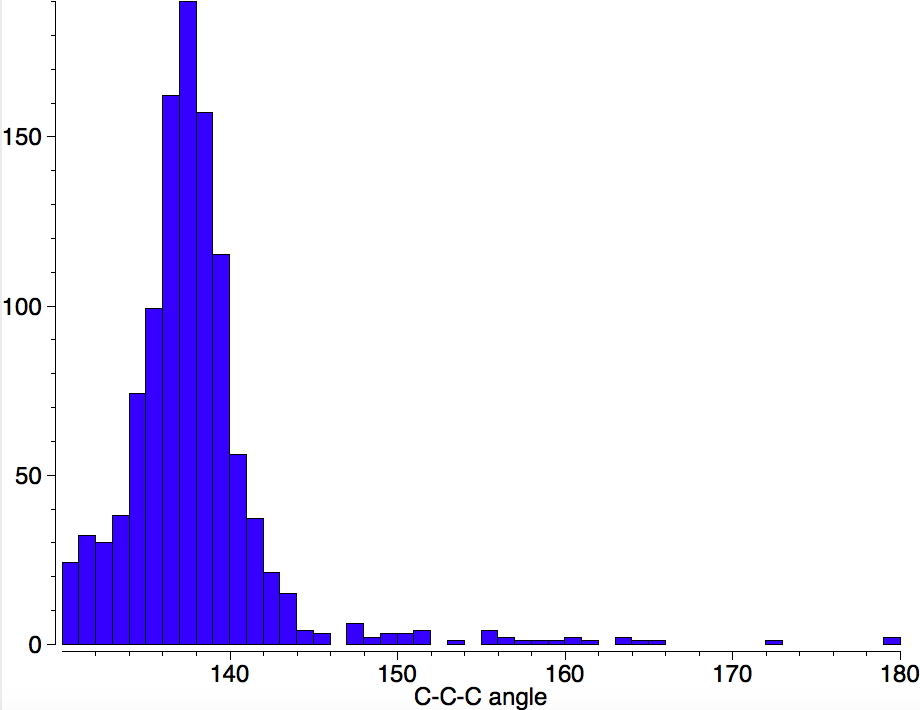
I am now inverting the previous question by asking what is the largest angle subtended at a chain of three connected 4-coordinate carbon atoms? Let’s see if further interesting chemistry can be unearthed.
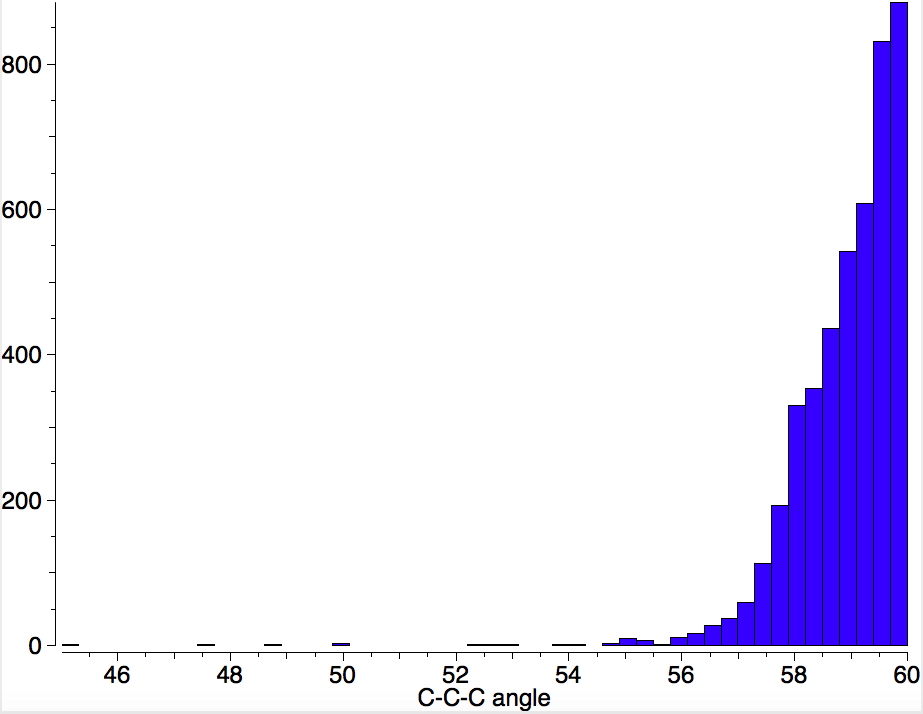
Is asking a question such as “what is the smallest angle subtended at a chain of three connected 4-coordinate carbon atoms” just seeking another chemical record, or could it unearth interesting chemistry?