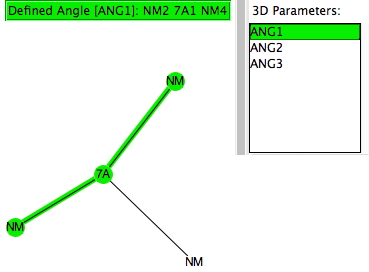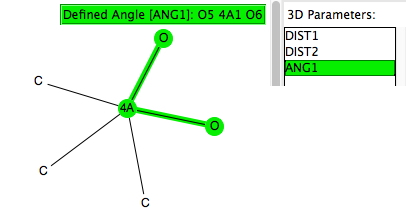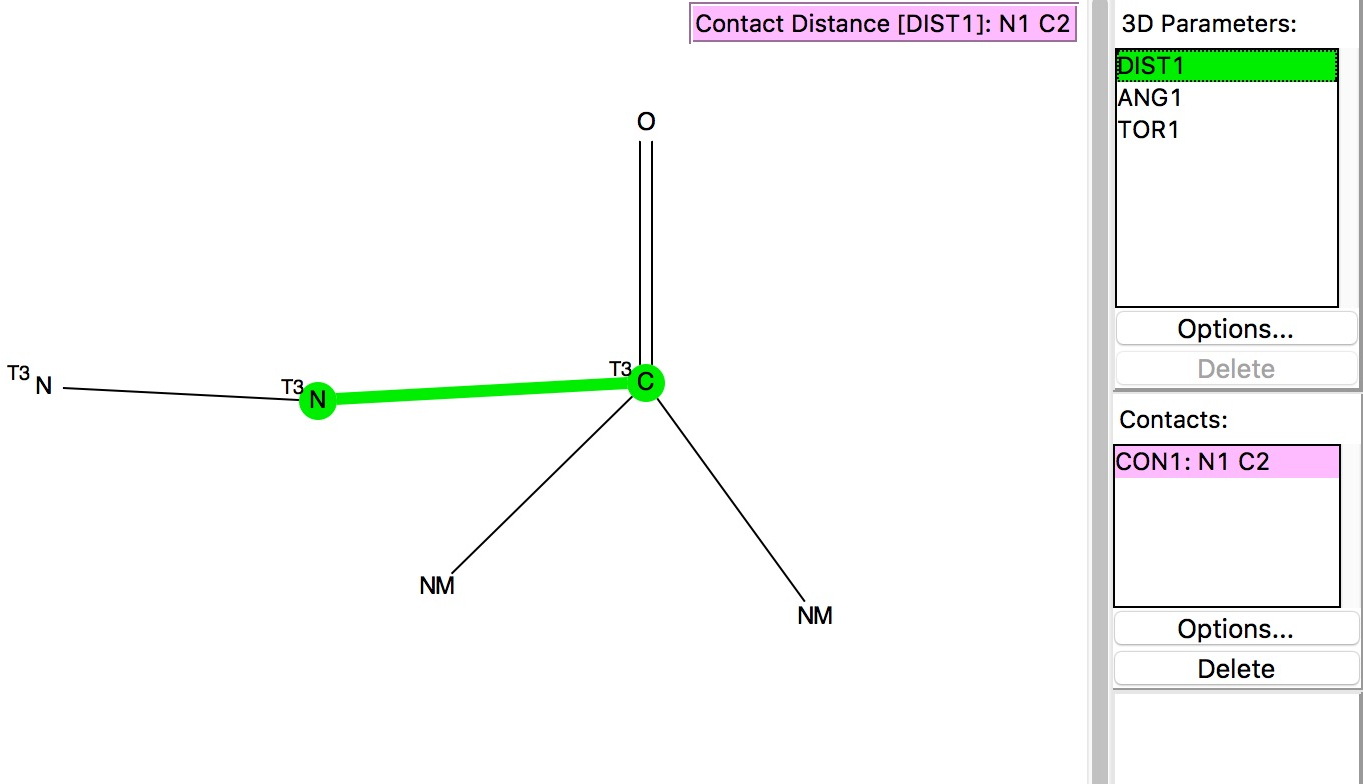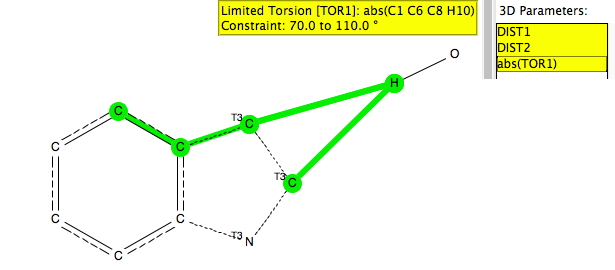
This is a follow-up to the post on exploring the directing influence of (electron donating) substituents on benzene[cite]10.1021/acs.jchemed.5b00346[/cite] with the focus on heteroaromatic rings such indoles, pyrroles and group 16 analogues (furans, thiophenes etc). The search query is shown above (and is available here[cite]10.14469/hpc/665[/cite]). As before, the distance is compared from an electrophile, modelled as