Management of research (data) outputs is a hot topic in the UK at the moment, although the topic has been rumbling for five years or more.

This post is prompted by the appearance of a retrospective special issue of C&E news, with what appears to be its very own Website: internet.cenmag.org.
I recently received two emails each with a subject line new approaches to research reporting. The traditional 350 year-old model of the (scientific) journal is undergoing upheavals at the moment with the introduction of APCs (article processing charges), a refereeing crisis and much more. Some argue that brand new thinking is now required.

I recently followed this bloggers trail; link1 → link2 to arrive at this delightful short commentary on atom-atom bonds in crystals by Jack Dunitz. Here he discusses that age-old question (to chemists), what is a bond? Even almost 100 years after Gilbert Lewis’ famous analysis, we continue to ponder this question.
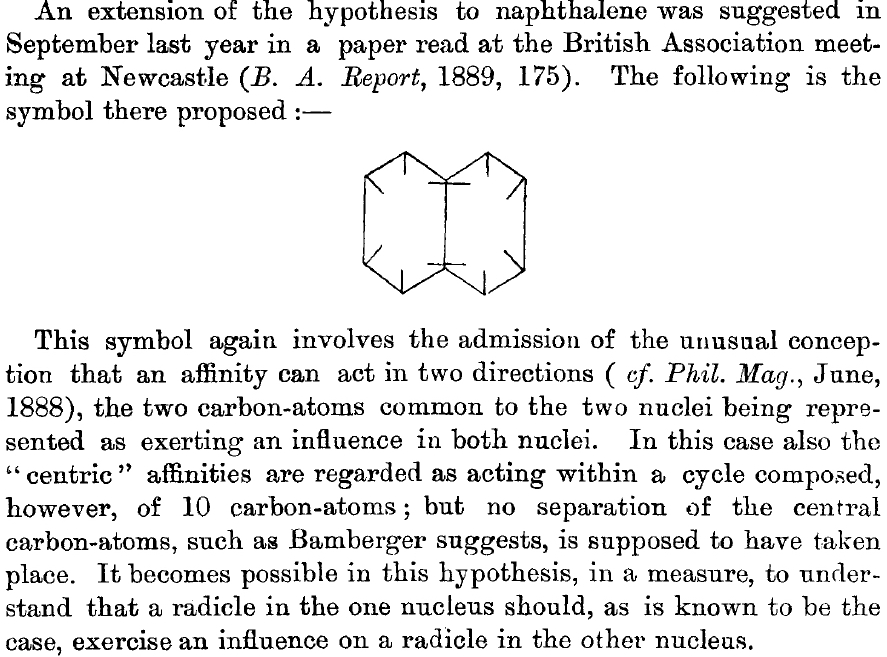
This is a little historical essay into the electronic structure of naphthalene, presented as key dates (and also collects comments made which were appended to other posts).
Previously on the kinetic isotope effects for the Baeyer-Villiger reaction, I was discussing whether a realistic computed model could be constructed for the mechanism. The measured KIE or kinetic isotope effects (along with the approximate rate of the reaction) were to be our reality check.
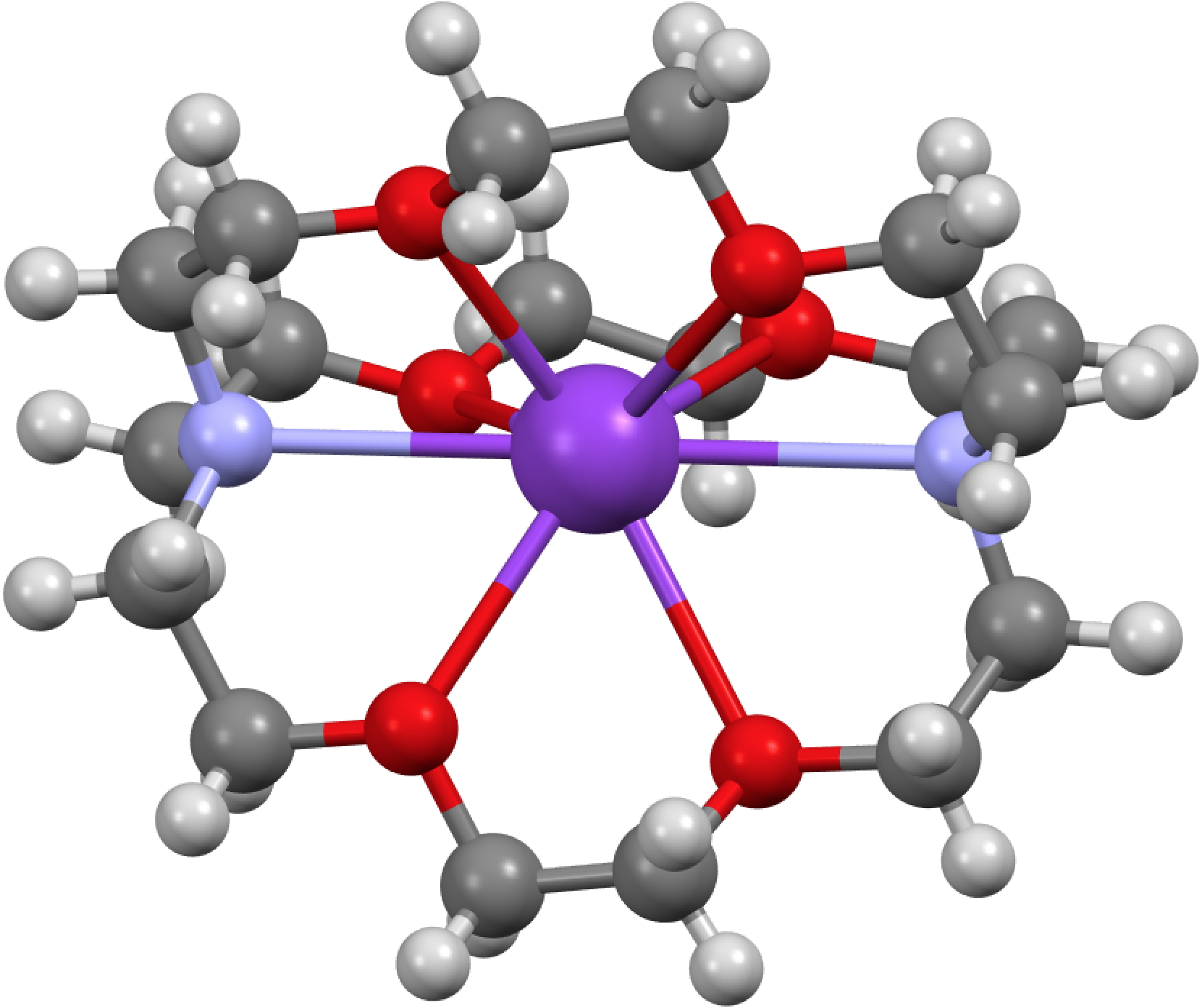
Peter Edwards has just given the 2015 Hofmann lecture here at Imperial on the topic of solvated electrons. An organic chemist knows this species as “e–” and it occurs in ionic compounds known as electrides; chloride = the negative anion of a chlorine atom, hence electride = the negative anion of an electron.
Recollect this earlier post on the topic of the Baeyer-Villiger reaction. In 1999 natural abundance kinetic isotope effects were reported and I set out to calculate the values predicted for a particular model constructed using Quantum mechanics. This comparison of measurement and calculation is nowadays a standard verification of both experiment and theory.
Open principles in the sciences in general and chemistry in particular are increasingly nowadays preached from funding councils down, but it can be more of a challenge to find innovative practitioners. Part of the problem perhaps is that many of the current reward systems for scientists do not always help promote openness.
The university sector in the UK has quality inspections of its research outputs conducted every seven years, going by the name of REF or Research Excellence Framework. The next one is due around 2020, and already preparations are under way! Here I describe how I have interpreted one of its strictures;
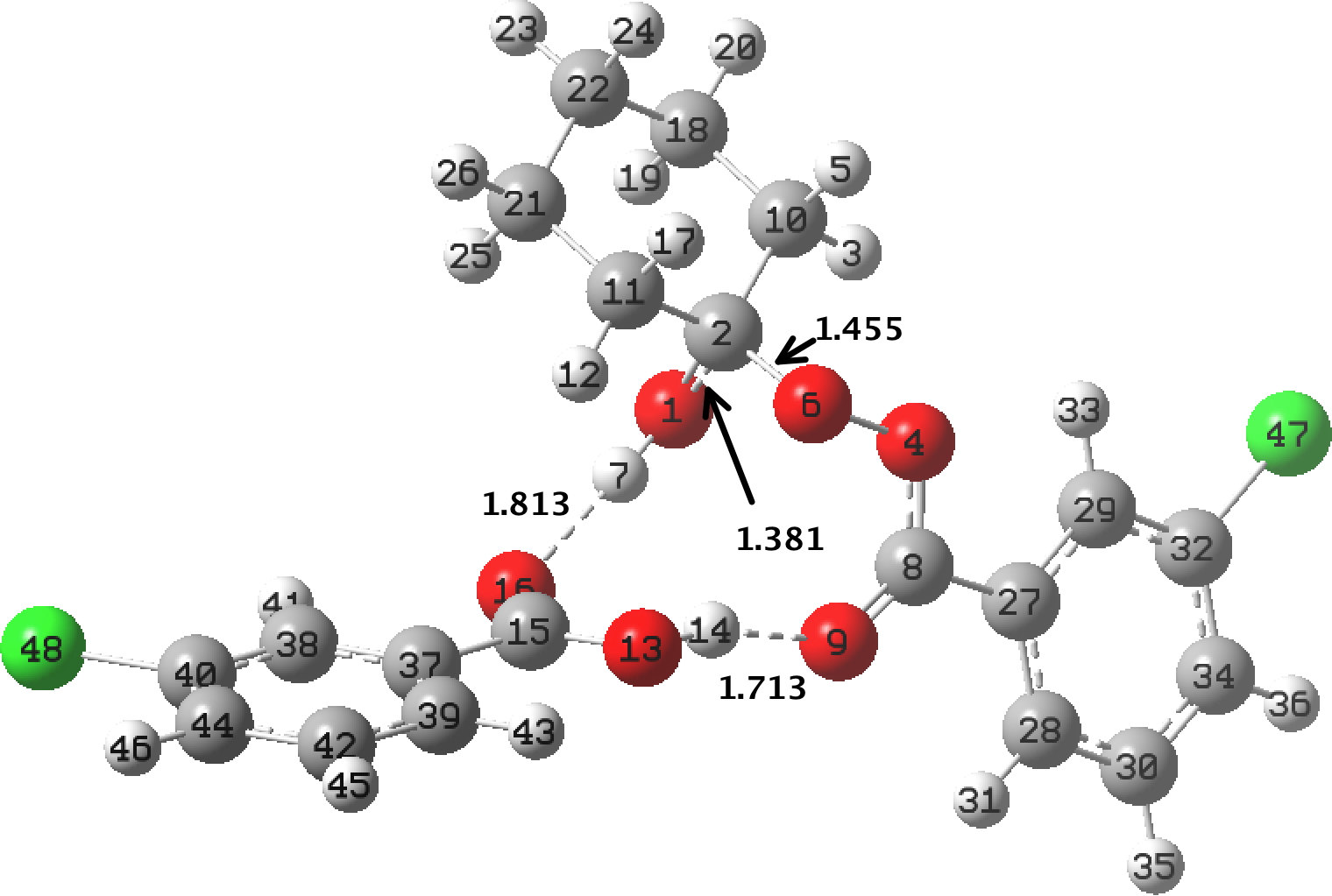
In the preceding post, I discussed the reaction between mCPBA (meta-chloroperbenzoic acid) and cyclohexanone, resulting in Baeyer-Villiger oxidation via a tetrahedral intermediate (TI). Dan Singleton, in whose group the original KIE (kinetic isotope measurements) were made, has kindly pointed out on this blog that his was a mixed-phase reaction, and that mechanistic comparison with homogenous solutions may not be […]