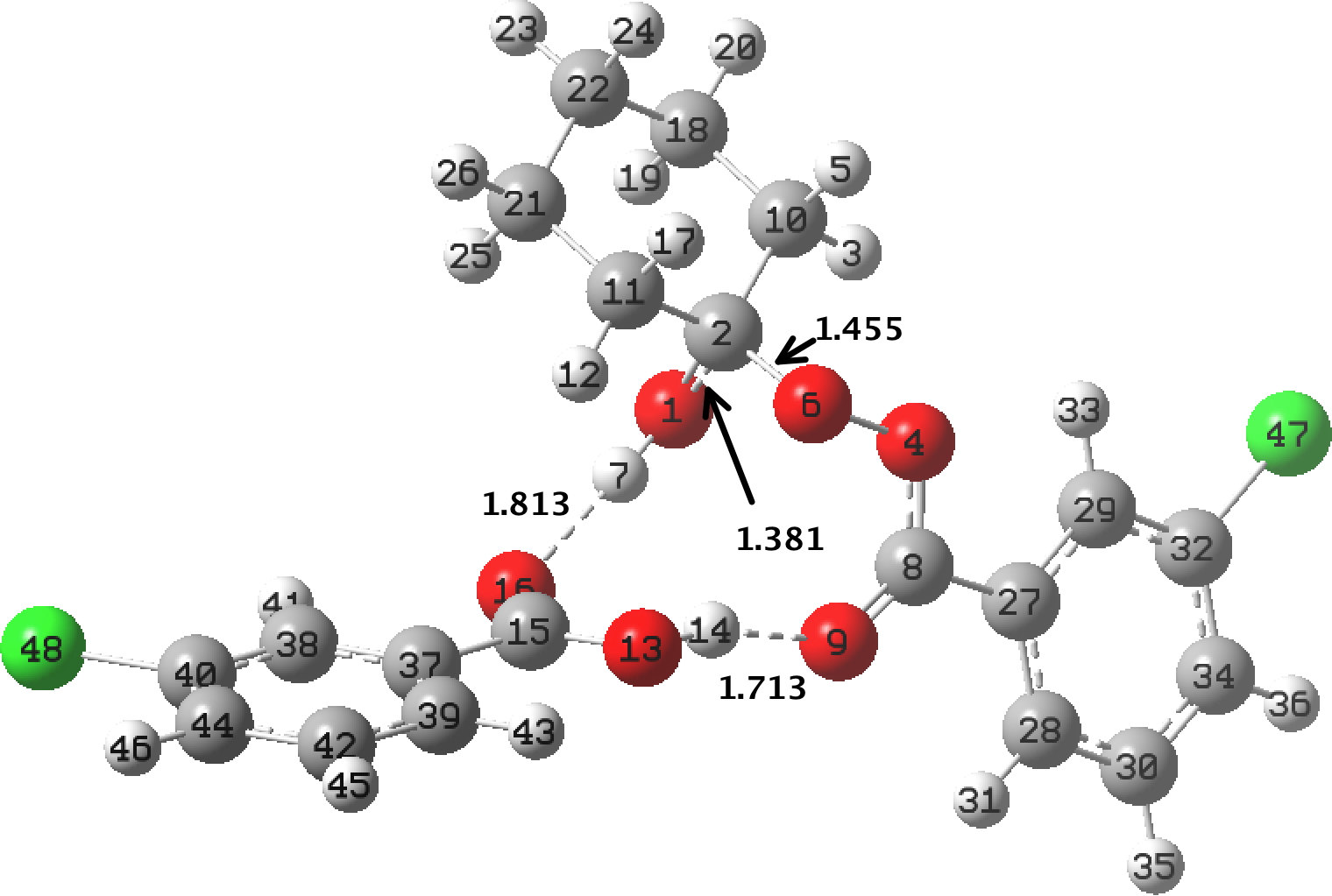
In the preceding post, I discussed the reaction between mCPBA (meta-chloroperbenzoic acid) and cyclohexanone, resulting in Baeyer-Villiger oxidation via a tetrahedral intermediate (TI). Dan Singleton, in whose group the original KIE (kinetic isotope measurements) were made, has kindly pointed out on this blog that his was a mixed-phase reaction, and that mechanistic comparison with homogenous solutions may not be […]