No doubt answers to the question posed in the previous post are already being obtained by experiment. Just in case that does not emerge in the next day or so, I offer a prediction here.

No doubt answers to the question posed in the previous post are already being obtained by experiment. Just in case that does not emerge in the next day or so, I offer a prediction here.
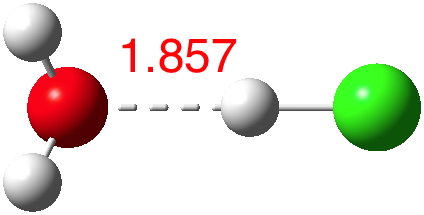
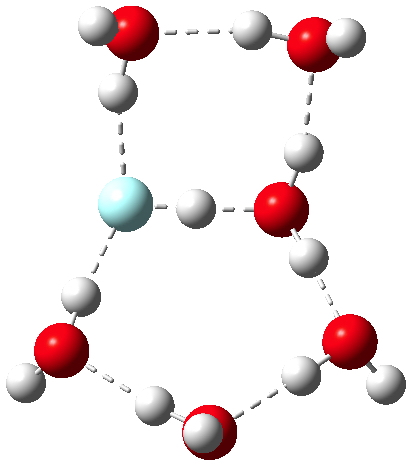
According to Guggemos, Slavicek and Kresin, about 5-6!. This is one of those simple ideas, which is probably quite tough to do experimentally. It involved blasting water vapour through a pinhole, adding HCl and measuring the dipole-moment induced deflection by an electric field. They found “evidence for a noticeable rise in the dipole moment occurring at n≈5–6“.
The title of this post refers to the site http://howopenisit.org/ which is in effect a license scraper for journal articles.
Steganone is an unusual natural product, known for about 40 years now. The assignment of its absolute configurations makes for an interesting, on occasion rather confusing, and perhaps not entirely atypical story. I will start with the modern accepted stereochemical structure of this molecule, which comes in the form of two separately isolable atropisomers.
The journal of chemical education has many little gems providing inspiration for laboratory experiments. Jonathan Piard reports one based on the reaction below; here I investigate the mechanism of this transformation.
Sometimes you come across a bond in chemistry that just shouts at you. This happened to me in 1989 with the molecule shown below. Here is its story and, 26 years later, how I responded.

Derek Lowe in his In the Pipeline blog is famed for spotting unusual claims in the literature and subjecting them to analysis. This one is entitled Odd Structures, Subjected to Powerful Computations. He looks at this image below, and finds the structures represented there might be a mistake, based on his considerable experience of these kinds of molecules. I expect he had a gut feeling within seconds of seeing the diagram.
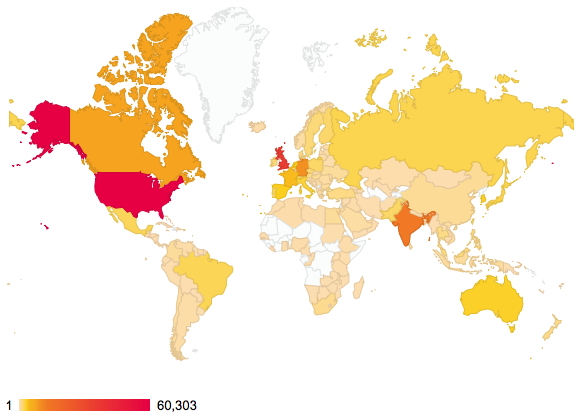
About two years ago, I posted on the distribution of readership of this blog. The passage of time has increased this from 144 to 176 countries. There are apparently between 189-196 such, so not quite yet complete coverage! Of course, it is the nature of the beast that whilst we can track countries, very little else is known about such readerships.

I started chemistry with a boxed set in 1962. In those days they contained serious amounts of chemicals, but I very soon ran out of most of them. Two discoveries turned what might have been a typical discarded christmas present into a lifelong career and hobby. The first was 60 Stoke Newington High Street in north London, the home of Albert N. Beck, Chemist (or his son;
I have written earlier about the Amsterdam Manifesto. That arose out of a conference on the theme of “ beyond the PDF ”, with one simple question at its heart: what can be done to liberate data from containers it was not designed to be in ? The latest meeting on this topic will happen in January 2015 as FORCE2015. The format is suitably modern, starting with a Hackathon, and then two days of talks, posters and demos.
These posts contain the computed potential energy surfaces for a fair few “text-book” reactions. Here I chart the course of the cyclopropanation of alkenes using the Simmons-Smith reagent,[cite]10.1021/ja01552a080[/cite] as prepared from di-iodomethane using zinc metal insertion into a C-I bond. Two reactions it can be compared with are the epoxidation of ethene using a peracid and dichlorocyclopropanation.