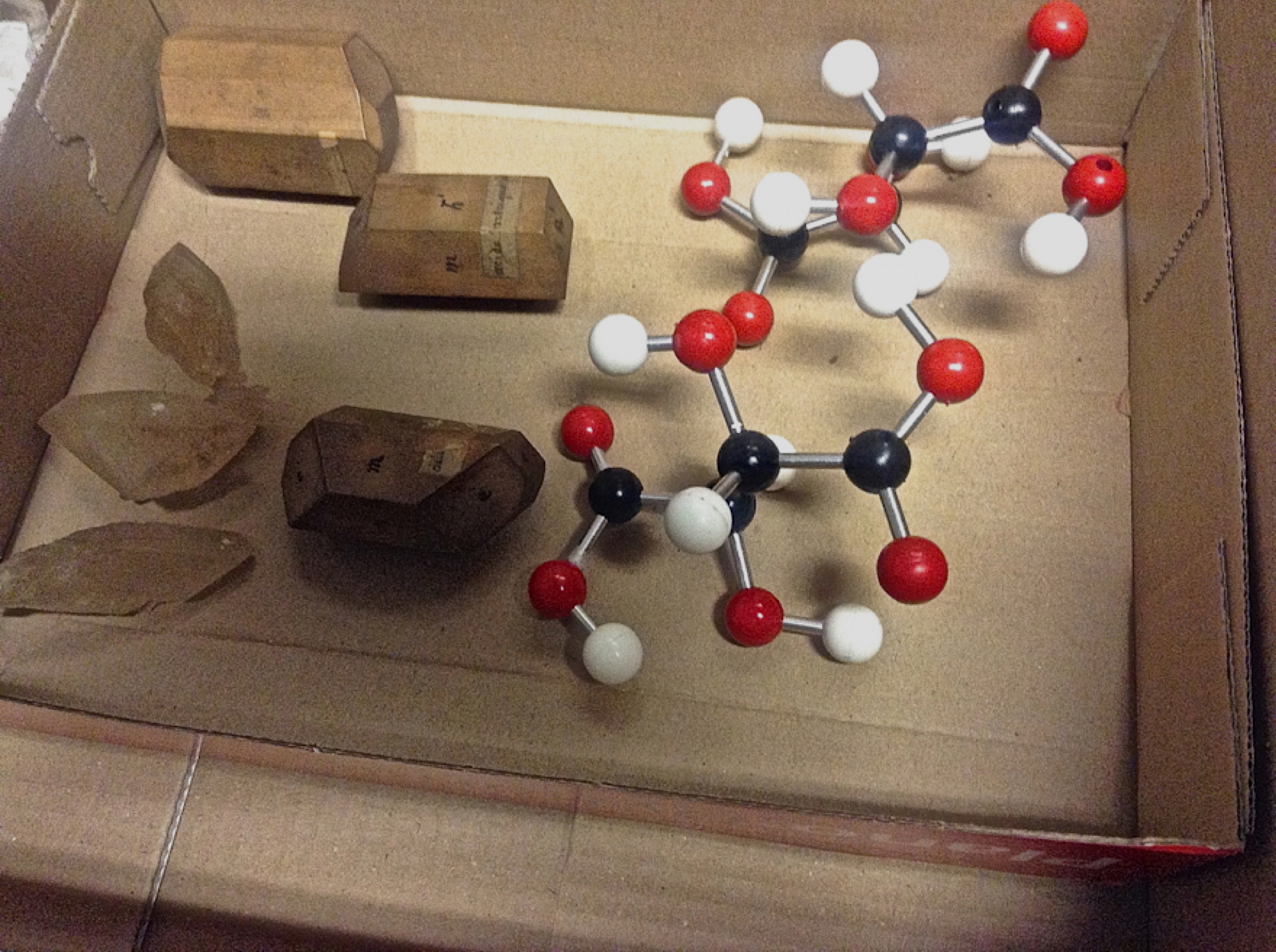
Not a computer in sight! I refer to a chemistry lab from the 1800s I was recently taken to, where famous french chemists such as Joseph Gay-Lussac, Michel Chevreul and Edmond Fremy were professors. Although not used for chemistry any more, it is an incredible treasure trove of objects. Here are photos of some.