Paul Schleyer sent me an email about a pattern he had spotted, between my post on F3SSF and some work he and Michael Mauksch had done 13 years ago with the intriguing title “Demonstration of Chiral Enantiomerization in a Four-Atom Molecule“. Let me explain the connection, but also to follow-up further on what I discovered in […]
I do go on rather a lot about enabling or hyper-activating data. So do others. Why is sharing data important?
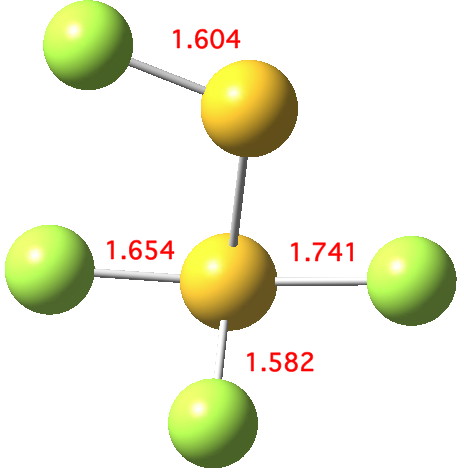
Andy Extance at the Chemistry World blog has picked up on a fascinating article on the dimer of SF2. This molecule has three F atoms on one S, and only one on the other; FSSF3. But all four S-F bonds are of different length.
According to Herges, the mechanism of single-step (concerted) reactions can be divided into three basic types; linear (e.g. substitution, elimination etc), pericyclic (e.g. Diels Alder) and a third much rarer, and hence very often overlooked type that was named coarctate.
The concept of a “hidden intermediate” in a reaction pathway has been promoted by Dieter Cremer and much invoked on this blog. When I used this term in a recent article of ours, a referee tried to object, saying it was not in common use in chemistry. The term clearly has an image problem.

We have been experimenting with full-colour 3D printing of molecular objects. I thought I might here share some of our observations.
The ultimate reduction in size for an engineer is to a single molecule. It’s been done for a car; now it has been reported for the pixel (picture-element).
The Amsterdam manifesto espouses the principles of citable open data.
Valence shell electron pair repulsion theory is a simple way of rationalising the shapes of many compounds in which a main group element is surrounded by ligands. ClF3 is a good illustration of this theory.
This potential example of a molecule on the edge of chaos was suggested to me by a student (thanks Stephen!), originating from an inorganic tutorial. It represents a class of Mo-complex ligated by two dithiocarbamate ligands and two aryl nitrene ligands (Ar-N:).
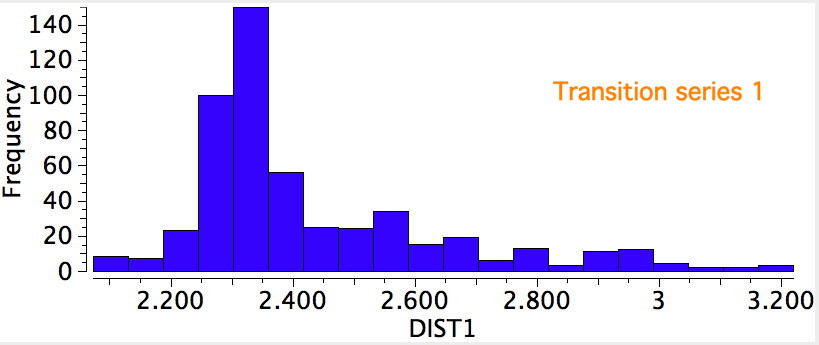
I noted previously that some 8-ring cyclic compounds could exist in either a planar-aromatic or a non-planar-non-aromatic mode, the mode being determined by apparently quite small changes in a ring substituent. Hunting for other examples of such chemistry on the edge, I did a search of the Cambridge crystal database for metal sulfides.