In a previous post on the topic, I remarked how the regiospecific ethanolysis of propene epoxide could be quickly and simply rationalised by inspecting the localized NBO orbital calculated for either the neutral or the protonated epoxide.
A few posts back, I explored the “benzidine rearrangement” of diphenyl hydrazine. This reaction requires diprotonation to proceed readily, but we then discovered that replacing one NH by an O as in N,O-diphenyl hydroxylamine required only monoprotonation to undergo an equivalent facile rearrangement.
I recently got an email from a student asking about the best way of rationalising epoxide ring opening using some form of molecule orbitals. This reminded me of the famous experiment involving propene epoxide.
This is another in the occasional series of “what a neat molecule”. In this case, more of a “what a neat idea”. The s-triazine below, when coordinated to eg ZnI2, forms what is called a metal-organic-framework, or MOF.
A recent theme here has been to subject to scrutiny well-known mechanisms supposedly involving intermediates. These transients can often involve the creation/annihilation of charge separation resulting from proton transfers, something that a cyclic mechanism can avoid. Here I revisit the formation of an oxime from hydroxylamine and propanone, but with one change.
Back in the days (1893) when few compounds were known, new ones could end up being named after the discoverer. Thus Feist is known for the compound bearing his name;
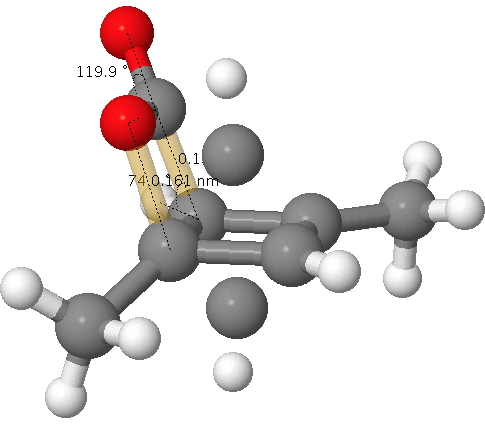
My previous dissection of the mechanism for ester hydrolysis dealt with the acyl-oxygen cleavage route (red bond). There is a much rarer alternative: alkyl-oxygen cleavage (green bond) which I now place under the microscope.
The mechanism of ester hydrolysis is a staple of examination questions in organic chemistry. To get a good grade, one might have to reproduce something like the below. Here, I subject that answer to a reality check.
The concept of a shared electron bond and its property of an order is almost 100 years old in modern form, when G. N. Lewis suggested a model for single and double bonds that involved sharing either 2 or 4 electrons between a pair of atoms.
My two previous explorations of aromatic substitutions have involved an electrophile (NO+ or Li+). Time now to look at a nucleophile, representing nucleophilic aromatic substitution. The mechanism of this is thought to pass through an intermediate analogous to the Wheland for an electrophile, this time known as the Meisenheimer complex.
A quartet of articles has recently appeared on the topic of cyclobutadiene.,,,. You will find a great deal discussed there, but I can boil it down to this essence.